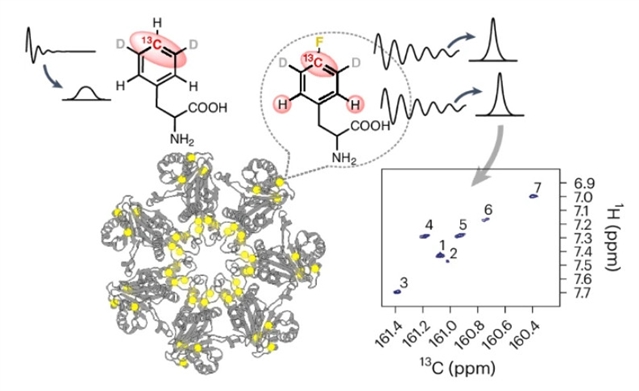
近日,美国哈佛医学院Haribabu Arthanari团队实现了利用4-19F-苯丙氨酸中弛豫优化的1H-13CF相关性作为探测大蛋白质结构和动力学的原子信标。该研究于2025年5月5日发表在《自然-化学》杂志上。
生物分子的核磁共振波谱为其结构、动力学和与结合伴侣的相互作用提供了原子水平的信息。然而,由快速弛豫和信号重叠引起的谱线展宽引起的信号衰减往往限制了核磁共振在大分子系统中的应用。研究组利用芳香族19F-13C自旋对中附着在19F上的13C核的慢弛豫特性,以及氟化13C核和间位氢原子之间的自旋-自旋耦合,记录二维1H-13CF相关光谱,并对13CF进行横向弛豫优化光谱选择。
为了实现这一目标,研究组合成了[4-19F13Cζ;3,5-2H2ε]Phe,设计用于优化弛豫特性,并采用了一种残基特异性途径将该残基全局掺入蛋白质中,以及一种位点特异性的4-19F Phe编码策略。这种方法使蛋白质的线宽变窄,范围从30 kDa至180 kDa,无需专门的19F兼容探针即可与小分子配体进行相互作用研究。
附:英文原文
Title: Leveraging relaxation-optimized 1H–13CF correlations in 4-19F-phenylalanine as atomic beacons for probing structure and dynamics of large proteins
Author: Boeszoermenyi, Andras, Radeva, Denitsa L., Schindler, Sebastian, Valadares, Veronica, Padmanabha Das, Krishna M., Dubey, Abhinav, Viennet, Thibault, Schmitt, Max, Kast, Peter, Gelev, Vladimir M., Stoyanov, Nikolay, Burdzhiev, Nikola, Petrov, Ognyan, Ficarro, Scott, Marto, Jarred, Geffken, Ezekiel A., Dhe-Paganon, Sirano, Seo, Hyuk-Soo, Alexander, Nathan D., Cooley, Richard B., Mehl, Ryan A., Kovacs, Helena, Anklin, Clemens, Bermel, Wolfgang, Kuprov, Ilya, Takeuchi, Koh, Arthanari, Haribabu
Issue&Volume: 2025-05-05
Abstract: NMR spectroscopy of biomolecules provides atomic level information into their structure, dynamics and interactions with their binding partners. However, signal attenuation from line broadening caused by fast relaxation and signal overlap often limits the application of NMR to large macromolecular systems. Here we leverage the slow relaxation properties of 13C nuclei attached to 19F in aromatic 19F–13C spin pairs as well as the spin–spin coupling between the fluorinated 13C nucleus and the hydrogen atom at the meta-position to record two-dimensional 1H–13CF correlation spectra with transverse relaxation-optimized spectroscopy selection on 13CF. To accomplish this, we synthesized [4-19F13Cζ; 3,5-2H2ε] Phe, engineered for optimal relaxation properties, and adapted a residue-specific route to incorporate this residue globally into proteins and a site-specific 4-19F Phe encoding strategy. This approach resulted in narrow linewidths for proteins ranging from 30kDa to 180kDa, enabling interaction studies with small-molecule ligands without requiring specialized 19F-compatible probes.
DOI: 10.1038/s41557-025-01818-8
Source: https://www.nature.com/articles/s41557-025-01818-8
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
