浙江大学梅清清团队近日实现了二氧化氮催化聚苯乙烯高选择性需氧氧化制苯甲酸。相关论文于2025年5月29日发表在《科学通报》杂志上。
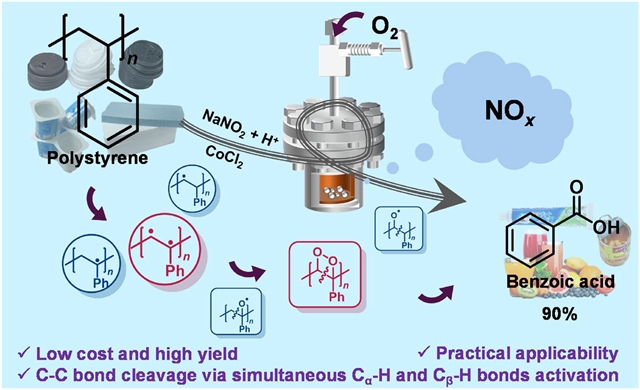
聚苯乙烯(PS)固有的稳定性和耐用性使其成为一种有价值的材料,但也使其化学回收变得复杂。通过氢原子转移(HAT)进行氧化降解是一种有前景的PS回收途径,但现有方法对目标产物的选择性不足。研究组展示了一种有效的方法,可以选择性地将PS转化为苯甲酸(BA),产率高达90%。
该工艺使用由亚硝酸钠和乙酸原位产生的二氧化氮(NO2)作为主要催化剂,在温和条件下(180°C,2巴(1巴=101千帕)氧气)使用市售氯化钴(CoCl2)作为助催化剂。机理研究表明,NO2通过HAT机制同时与苄基的Cα-H和Cβ-H键相互作用,形成二氧杂环丁烷中间体。该中间体经历开环过程,促进C-C键断裂并随后氧化为BA。此外,现实世界PS废物的适用性凸显了该过程在推进PS回收技术方面的潜力,最终有助于减轻塑料废物对环境的影响。
附:英文原文
Title: Highly selective aerobic oxidation of polystyrene to benzoic acid catalyzed by nitrogen dioxide
Author: Qingqing Mei a b
Issue&Volume: 2025/05/29
Abstract: The inherent stability and durability of polystyrene (PS) make it a valuable material but also complicate its chemical recycling. Oxidative degradation via hydrogen atom transfer (HAT) is a promising route for PS recovery, yet existing methods suffer from insufficient selectivity toward target products. Herein, we demonstrate an efficient approach to selectively convert PS into benzoic acid (BA) with an impressive yield of 90%. The process uses nitrogen dioxide (NO2), generated in situ from sodium nitrite and acetic acid, as the primary catalyst, with commercially available cobalt chloride (CoCl2) as a cocatalyst under mild conditions (180 °C, 2 bar (1 bar = 101 kPa) O2). Mechanistic studies revealed that NO2 simultaneously interacts with the Cα–H and Cβ–H bonds of the benzyl group via a HAT mechanism to form a dioxetane intermediate. This intermediate undergoes a ring-opening process, facilitating C–C bond cleavage and subsequent oxidation to BA. Furthermore, the demonstrated applicability of real-world PS waste underscores the potential of this process to advance PS recycling technologies, ultimately helping mitigate the environmental impact of plastic waste.
DOI: 10.1016/j.scib.2025.05.034
Source: https://www.sciencedirect.com/science/article/abs/pii/S2095927325005742
Science Bulletin:《科学通报》,创刊于1950年。隶属于SciEngine出版平台,最新IF:18.9
官方网址:https://www.sciengine.com/SB/home
投稿链接:https://mc03.manuscriptcentral.com/csb
