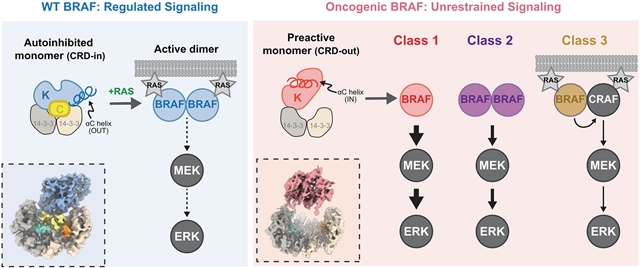
通过冷冻电镜,研究组解决了三个致癌BRAF突变类的三维结构,包括常见的V600E变体。这些突变破坏了野生型BRAF的自抑制状态,由富含半胱氨酸的结构域和激酶结构域之间的相互作用介导,从而将激酶结构域转变为预激活的构象。这种结构变化可能是由螺旋αC位移引起的。PLX8394是一种BRAF抑制剂,它稳定了螺旋αC的非活性构象,恢复了致癌BRAF的自抑制构象,解释了这类化合物的特性。
据了解,大鼠肉瘤(RAS) -细胞外信号调节激酶(ERK)途径的不受控制的激活驱动肿瘤生长,通常成为致癌BRAF突变的主题。BRAF的调控,包括单体自抑制和二聚化激活,已被严格审查,但使致癌突变体逃避调控的机制仍不清楚。
附:英文原文
Title: BRAF oncogenic mutants evade autoinhibition through a common mechanism
Author: Hugo Lavoie, Ting Jin, Driss Lajoie, Marion Decossas, Patrick Gendron, Bing Wang, Frantisek Filandr, Malha Sahmi, Chang Hwa Jo, Sandra Weber, Geneviève Arseneault, Sasmita Tripathy, Pierre Beaulieu, Doris A. Schuetz, David C. Schriemer, Anne Marinier, William J. Rice, Pierre Maisonneuve, Marc Therrien
Issue&Volume: 2025-05-29
Abstract: Uncontrolled activation of the rat sarcoma (RAS)–extracellular signal–regulated kinase (ERK) pathway drives tumor growth, often because of oncogenic BRAF mutations. BRAF regulation, involving monomeric autoinhibition and activation by dimerization, has been intensely scrutinized, but mechanisms enabling oncogenic mutants to evade regulation remain unclear. By using cryo–electron microscopy, we solved the three-dimensional structures of the three oncogenic BRAF mutant classes, including the common V600E variant. These mutations disrupted wild-type BRAF's autoinhibited state, mediated by interactions between the cysteine-rich domain and kinase domain, thereby shifting the kinase domain into a preactivated conformation. This structural change likely results from helix αC displacement. PLX8394, a BRAF inhibitor that stabilizes helix αC in an inactive conformation, restored the autoinhibited conformation of oncogenic BRAF, explaining the properties of this class of compounds.
DOI: adp2742
Source: https://www.science.org/doi/10.1126/science.adp2742
