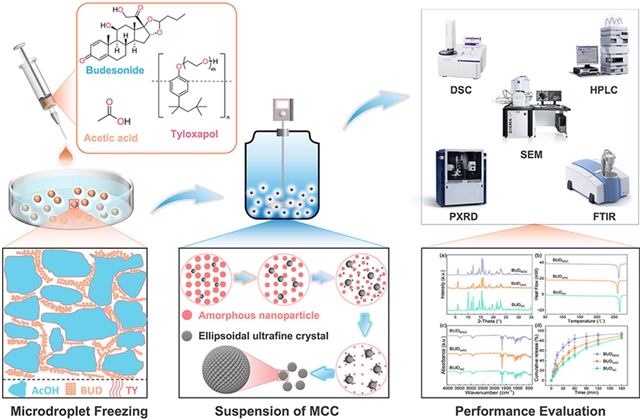
天津大学Zhenguo Gao团队近日实现了微滴冷冻结晶法制备物理化学性能最佳的布地奈德微粒。相关论文于2025年5月27日发表在《颗粒学报》杂志上。
吸入疗法是治疗肺部疾病的关键,但其疗效在很大程度上取决于药物颗粒的物理化学性质。本研究介绍了一种新的微滴冷冻结晶(MCC)技术来制备可吸入的布地奈德(BUD)颗粒。MCC工艺结合了在液氮中快速低温冷冻载药微滴,然后在反溶剂环境中进行添加剂引导的悬浮结晶。低温冷冻抑制分子流动并防止聚集,保持溶质分布均匀。随后在反溶剂系统中的受控结晶能够精确定制纳米粒子形态,同时避免过饱和驱动的非晶化。
系统优化确定了MCC条件,产生体积中值直径为3.0μm、球度>94%、结晶度>98%、吸湿性最小(<0.5%)的BUD超细晶体。与传统的空气喷射研磨BUD(约90%结晶度和约3%吸湿性)相比,MCC工程颗粒的物理化学稳定性和溶解性能显著提高(180分钟内为94%)。MCC策略将低温冷冻和相变解耦,避免了自上而下的限制(如研磨诱导的非晶化)和自下而上的问题(不受控制的成核/聚集),以实现可吸入药物颗粒的可扩展和高度精确的生产。
附:英文原文
Title: Microdroplet cryo-crystallization for producing budesonide microparticles with optimized physicochemical properties
Author: Zhenguo Gao
Issue&Volume: 2025/05/27
Abstract: Inhalation therapies are pivotal for treating pulmonary diseases, yet their efficacy critically depends on the physicochemical properties of drug particles. This study introduces a novel microdroplet cryo-crystallization (MCC) technique to fabricate inhalable budesonide (BUD) particles. The MCC process combines rapid cryogenic freezing of drug-loaded microdroplets in liquid nitrogen, followed by additive-guided suspension crystallization in an anti-solvent environment. Cryogenic freezing suppresses molecular mobility and prevents aggregation, preserving uniform solute distribution. Subsequent controlled crystallization in the anti-solvent system enables precise tailoring of nanoparticle morphologies while avoiding supersaturation-driven amorphization. Systematic optimization identified MCC conditions yielding BUD ultrafine crystals with a volume median diameter of 3.0 μm, >94% sphericity, >98% crystallinity, and minimal hygroscopicity (<0.5%). Compared to conventional air-jet milled BUD (~90% crystallinity and ~3% hygroscopicity), the MCC-engineered particles exhibit significantly improved physicochemical stability and dissolution performance (94% in 180 min). The MCC strategy decouples cryogenic freezing and phase transformation, avoiding top-down limitations (e.g., milling-induced amorphization) and bottom-up issues (uncontrolled nucleation/aggregation) to achieve scalable and highly precise production of inhalable drug particles.
DOI: 10.1016/j.partic.2025.05.014
Source: https://www.sciencedirect.com/science/article/abs/pii/S1674200125001476
Particuology:《颗粒学报》,创刊于2003年。隶属于爱思唯尔出版集团,最新IF:3.5
官方网址:https://www.sciencedirect.com/journal/particuology
投稿链接:https://www2.cloud.editorialmanager.com/partic/default2.aspx
