普渡大学John J. G. Tesmer研究小组取得一项新突破。他们报道了磷酸化条形码对抑制蛋白与趋化因子受体结合的影响。2025年5月21日出版的《自然》杂志发表了这项成果。
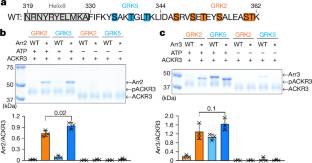
为了解决这个问题,研究小组开发了一种抗原结合片段(Fab7),它可以识别活性arrestin2 (β-arrestin1)和arrestin3 (β-arrestin2),而不与结合的受体多肽相互作用。该课题组研究人员对Fab7进行了主题研究,以确定这两种抑制蛋白在其C端尾部不同区域被GRK2或GRK5磷酸化的非典型趋化因子受体3 (ACKR3)复合物中的结构。GRK2磷酸化的ACKR3导致更多异质性的“尾部模式”组装,而GRK5磷酸化导致更刚性的“ACKR3相邻”组装。出乎意料的是,两种拦阻蛋白的指环都与胶束表面而不是受体细胞内袋结合,拦阻蛋白3更具动态性,部分原因是其缺乏膜锚定基元。它们所涉及的条形码区域和捕获蛋白同工型都可以改变GPCR-捕获蛋白复合物的结构和动力学,为独特的下游细胞效应提供了可能的机制基础,例如趋化因子清除的效率和ACKR3中捕获蛋白结合的灵活性。
据悉,通过不同的G蛋白偶联受体(GPCR)激酶(GRKs)安装在活性七跨膜受体的不同区域的独特磷酸化“条形码”已被提出,以促进不同的细胞结果,但目前尚不清楚是否或如何不同地参与这些条形码。
附:英文原文
Title: Effect of phosphorylation barcodes on arrestin binding to a chemokine receptor
Author: Chen, Qiuyan, Schafer, Christopher T., Mukherjee, Somnath, Wang, Kai, Gustavsson, Martin, Fuller, James R., Tepper, Katelyn, Lamme, Thomas D., Aydin, Yasmin, Agrawal, Parth, Terashi, Genki, Yao, Xin-Qiu, Kihara, Daisuke, Kossiakoff, Anthony A., Handel, Tracy M., Tesmer, John J. G.
Issue&Volume: 2025-05-21
Abstract: Unique phosphorylation ‘barcodes’ installed in different regions of an active seven-transmembrane receptor by different G-protein-coupled receptor (GPCR) kinases (GRKs) have been proposed to promote distinct cellular outcomes1, but it is unclear whether or how arrestins differentially engage these barcodes. Here, to address this, we developed an antigen-binding fragment (Fab7) that recognizes both active arrestin2 (β-arrestin1) and arrestin3 (β-arrestin2) without interacting with bound receptor polypeptides. We used Fab7 to determine the structures of both arrestins in complex with atypical chemokine receptor 3 (ACKR3) phosphorylated in different regions of its C-terminal tail by either GRK2 or GRK5 (ref.2). The GRK2-phosphorylated ACKR3 resulted in more heterogeneous ‘tail-mode’ assemblies, whereas phosphorylation by GRK5 resulted in more rigid ‘ACKR3-adjacent’ assemblies. Unexpectedly, the finger loops of both arrestins engaged the micelle surface rather than the receptor intracellular pocket, with arrestin3 being more dynamic, partly because of its lack of a membrane-anchoring motif. Thus, both the region of the barcode and the arrestin isoform involved can alter the structure and dynamics of GPCR–arrestin complexes, providing a possible mechanistic basis for unique downstream cellular effects, such as the efficiency of chemokine scavenging and the robustness of arrestin binding in ACKR3.
DOI: 10.1038/s41586-025-09024-9
Source: https://www.nature.com/articles/s41586-025-09024-9
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
