河南大学简永平小组在研究中取得进展。他们揭示了STING通过抑制CPT1A介导的脂肪酸β-氧化来抑制食管鳞状细胞癌。这一研究成果于2025年5月20日发表在国际顶尖学术期刊《中国药理学报》上。
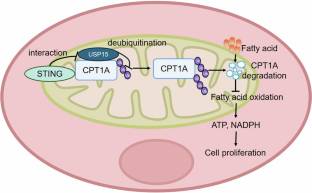
在本研究中,课题组研究了STING对ESCC中FAO和肿瘤发生的影响。该团队发现,与邻近正常组织相比,ESCC中STING的表达水平显著降低。在ESCC细胞系KYSE-510中,敲低STING显著提高脂质代谢物,减少细胞内脂滴,增加FAO产物,而过表达STING通过抑制FAO抑制ESCC细胞增殖和肿瘤进展。靶向脂质代谢组学分析显示,STING与肉毒碱棕榈酰转移酶1A (CPT1A)相互作用,CPT1A是粮农组织的一种关键酶。STING通过破坏CPT1A与USP15(一种去泛素化酶)的相互作用,促进CPT1A的泛素化和降解。CPT1A抑制剂依托莫西(50μM)可逆转KYSE-30细胞中STING缺失引起的FAO升高。在体外和体内模型中,补充棕榈酸可以恢复STING诱导的生长抑制,恢复肿瘤细胞的生长。此外,在4-NQO诱导的ESCC小鼠中,STING敲除导致肿瘤加速进展,这可以通过抑制CPT1A来缓解。他们的研究结果表明,减少STING表达可增强FAO并促进ESCC细胞增殖,这意味着抑制FAO可能是ESCC的潜在治疗策略。
据悉,食管鳞状细胞癌(ESCC)具有侵袭性高、预后差的特点。代谢重编程是ESCC的一个标志,脂质代谢经常上调。脂质代谢,特别是脂肪酸β氧化(FAO),在能量稳态、膜生物合成和肿瘤进展中起着至关重要的作用。干扰素基因刺激因子(STING)是一种关键的先天免疫信号分子,通过抑制己糖激酶2作为代谢检查点,从而限制有氧糖酵解,增强抗肿瘤免疫反应。
附:英文原文
Title: STING inhibits the progression of esophageal squamous cell carcinoma by suppressing CPT1A-mediated fatty acid β-oxidation
Author: Zhang, Lei, Zhu, Ling-jun, Zhao, Yuan, Lei, Xin-yuan, Wu, Dan-hui, He, Kai-yue, Liu, Meng-jie, Yang, Jing-yu, Guo, Jin-rong, Jiang, Zhi-hao, Yan, Zhen-hua, Xu, Zhi-xiang, Jian, Yong-ping
Issue&Volume: 2025-05-20
Abstract: Esophageal squamous cell carcinoma (ESCC) is characterized by high aggressiveness and poor prognosis. Metabolic reprogramming is a hallmark of ESCC, with lipid metabolism frequently upregulated. It has been shown that lipid metabolism, particularly fatty acid β-oxidation (FAO), plays an essential role in energy homeostasis, membrane biosynthesis, and tumor progression. Stimulator of interferon genes (STING), a key innate immune signaling molecule, also acts as a metabolic checkpoint by inhibiting hexokinase 2, thereby limiting aerobic glycolysis and enhancing anti-tumor immune responses. In this study, we investigated the impact of STING on FAO and tumorigenesis in ESCC. We showed that the expression levels of STING were significantly reduced in ESCC compared to adjacent normal tissue. In the ESCC cell line KYSE-510, knockdown of STING significantly elevated lipid metabolites, decreased intracellular lipid droplets, and increased FAO products, whereas overexpression of STING inhibited ESCC cell proliferation and tumor progression by suppressing FAO. Targeted lipid metabolomic analyses revealed that STING interacted with carnitine palmitoyltransferase 1A (CPT1A), a key enzyme in FAO. STING promoted the ubiquitination and degradation of CPT1A by disrupting its interaction with USP15, a deubiquitinating enzyme. Treatment with the CPT1A inhibitor etomoxir (50μM) reversed the increased FAO induced by STING depletion in KYSE-30 cells. In both in vitro and in vivo models, supplementation with palmitic acid rescued STING-induced growth inhibition, restoring tumor cell growth. In addition, STING knockout in 4-NQO-induced ESCC mice led to accelerated tumor progression, which could be mitigated by CPT1A inhibition. Our results suggest that reduced STING expression enhances FAO and promotes ESCC cell proliferation, implicating FAO suppression as a potential therapeutic strategy for ESCC.
DOI: 10.1038/s41401-025-01581-z
Source: https://www.nature.com/articles/s41401-025-01581-z
Acta Pharmacologica Sinica:《中国药理学报》,创刊于1980年。隶属于施普林格·自然出版集团,最新IF:8.2
官方网址:http://www.chinaphar.com/
投稿链接:https://mc.manuscriptcentral.com/aphs
