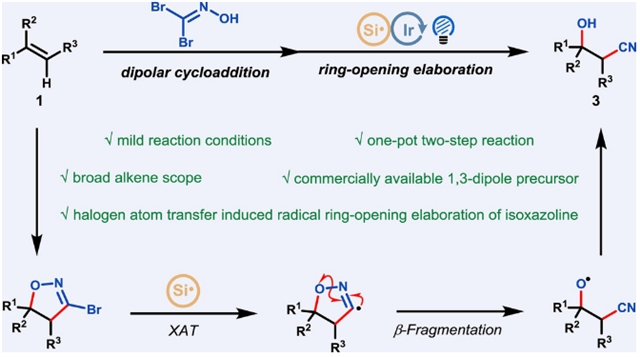
南京工业大学冯超团队实现了卤素原子转移诱导3-溴异恶唑啉环加合物自由基开环精化使烯烃氰化羟基化。相关论文发表在2025年5月19日出版的《中国化学》杂志上。
研究组提出了一种高效的一锅法、两步法合成β-羟基腈支架的方法,该方法从易于获得的烯烃开始,具有显著的合成价值和显著的生物活性。该方法在很大程度上依赖于高度区域选择性(3+2)环加成反应的无缝集成,该反应使用市售的1,1-二溴甲醛肟作为1,3-偶极前体,随后卤素原子转移诱导自由基开环,形成3-溴-2-异恶唑啉环加合物。
该方案的特点是反应条件温和,烯烃范围广,所获得的氰基羟基化产物可进行各种衍生化,为获得多官能化分子提供了一条通用且实用的途径。
附:英文原文
Title: Expedient Cyano-hydroxylation of Alkenes Enabled by Halogen Atom Transfer Induced Radical Ring-Opening Elaboration of 3-Bromo-isoxazoline Cycloadducts
Author: Hui Wang, Qing Chen, Shuhui Wang, Cheng-Qiang Wang, Chao Feng
Issue&Volume: 2025-05-19
Abstract: Herein, we present a highly efficient one-pot, two-step synthesis of β-hydroxy nitrile scaffolds, which possess both significant synthetic value and notable biological activity, starting from readily accessible alkenes. This methodology relies crucially on the seamless integration of a highly regioselective (3+2) cycloaddition reaction, employing the commercially available 1,1-dibromoformaldoxime as the 1,3-dipole precursor, with a subsequent halogen atom transfer-induced radical ring-opening elaboration of the resulting 3-bromo-2-isoxazoline cycloadducts. This protocol is featured by mild reaction conditions, broad alkene scope and various derivatizations of the obtained cyano-hydroxylation products, offering a versatile and practical pathway to accessing multi-functionalized molecules.
DOI: 10.1002/cjoc.70070
Source: https://onlinelibrary.wiley.com/doi/10.1002/cjoc.70070
Chinese Journal of Chemistry:《中国化学》,创刊于1983年。隶属于Wiley,最新IF:5.4
官方网址:https://onlinelibrary.wiley.com/journal/16147065
投稿链接:https://mc.manuscriptcentral.com/cjoc
