近日,天津大学张蕾团队研究了防冻肽的精准从头设计原理。2025年5月13日出版的《美国化学会杂志》发表了这项成果。
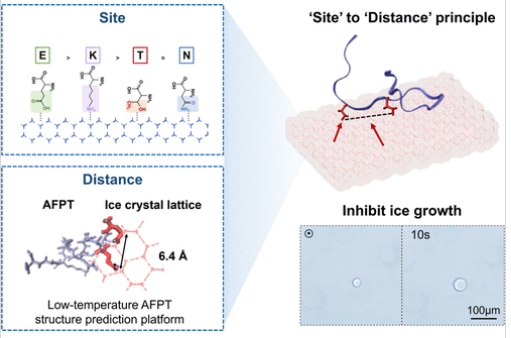
由于抗冻肽(AFPT)的活性结构尚未明确,从头设计AFPT是一项艰巨的挑战。研究组从理解AFPT的结构-活动关系的角度描述了AFPT从头设计的“站点到距离”原则。第一步是将E点(被确定为最有效的冰结合位点(IBS),其结合能至少是天然IBS的4倍)插入候选骨架。第二步,基于IBS(E),是依靠新建立的低温AFPT结构预测平台,明智地调整位点的距离,以匹配冰晶晶格的有利数量,实现最强的冰结合。
由此产生的AFPT显示出单晶生长速率的大幅降低,远优于>100个天然或设计的AFPT,包括所有已报道的AFPT。治疗细胞的冷冻保存进一步证实了这一设计原理的准确性。
附:英文原文
Title: Precise de novo Design Principle of Antifreeze Peptides
Author: Xiangyu Zhang, Jing Yang, Yunqing Tian, Lei Zhang
Issue&Volume: May 13, 2025
Abstract: De novo design of antifreeze peptides (AFPTs) represents a formidable challenge due to the unclarified active structure of AFPTs. Here, we describe a “Site to Distance” principle for de novo design of AFPTs, in terms of understanding their structure–activity relationships. The first step is to point E, identified as the most potent ice-binding site (IBS) possessing at least 4-fold binding energy than natural IBSs, into the candidate backbones. The second step, based on the IBS (E), is to judiciously adjust the distances of sites to match the favorable number of the ice crystal lattice to achieve the strongest ice-binding, relying on a newly established low-temperature AFPT structure prediction platform. The resultant AFPTs show a substantial reduction in single ice crystal growth rates, much superior to >100 natural or designed AFPTs, including all that have been reported. Cryopreservation of therapeutic cells further confirms the accuracy of this design principle.
DOI: 10.1021/jacs.4c18537
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.4c18537
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
