中国科学院深圳先进技术研究院甘海云研究团队宣布他们开发出肿瘤中染色体外DNA复制和维持与DNA损伤通路的耦合。该研究于2025年4月28日发表于国际一流学术期刊《细胞》杂志上。
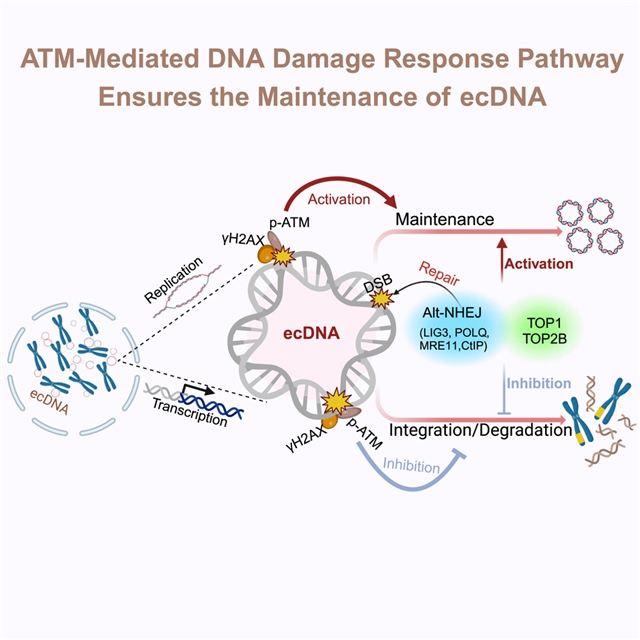
利用CRISPR-C技术,研究组建立了携带ecDNA (ecDNA+)的细胞模型。通过利用这些模型和其他成熟的系统,研究团队证明了ecDNA可以在ecDNA+细胞中复制和维持。ecDNA的复制激活共济失调毛细血管扩张突变(ATM)介导的DNA损伤反应(DDR)途径。拓扑异构酶,如TOP1和TOP2B,在ecDNA复制诱导的DNA双链断裂(DSBs)中发挥作用。这些升高的DSB的一个子集持续进入有丝分裂期,并主要通过选择性非同源末端连接(alt-NHEJ)途径修复,该途径涉及POLθ和LIG3。相应地,ecDNA的维持需要DDR,抑制DDR会损害ecDNA的循环。总之,该研究团队证明了ecDNA维持和DDR之间的相互作用,为ecDNA+肿瘤的检测和治疗提供了新的见解。
据了解,染色体外DNA (ecDNA)驱动癌细胞的进化。然而,ecDNA的功能意义及其参与其复制和维持的分子成分在很大程度上仍然未知。
附:英文原文
Title: Extrachromosomal DNA replication and maintenance couple with DNA damage pathway in tumors
Author: Xing Kang, Xinran Li, Jiaqi Zhou, Yang Zhang, Lingyu Qiu, Congcong Tian, Zhiwen Deng, Xiaoyan Liang, Ziwei Zhang, Songlin Du, Suili Hu, Nan Wang, Zhen Yue, Yajing Xu, Yuan Gao, Junbiao Dai, Zhiquan Wang, Chuanhe Yu, Jinyi Chen, Yuchun Wu, Liangming Chen, Yuan Yao, Sitong Yao, Xinran Yang, Lixia Yan, Qing Wen, Olivia M. Depies, Kuiming Chan, Xiaohuan Liang, Gang Li, Zhike Zi, Xiangyu Liu, Haiyun Gan
Issue&Volume: 2025-04-28
Abstract: Extrachromosomal DNA (ecDNA) drives the evolution of cancer cells. However, the functional significance of ecDNA and the molecular components involved in its replication and maintenance remain largely unknown. Here, using CRISPR-C technology, we generated ecDNA-carrying (ecDNA+) cell models. By leveraging these models alongside other well-established systems, we demonstrated that ecDNA can replicate and be maintained in ecDNA+ cells. The replication of ecDNA activates the ataxia telangiectasia mutated (ATM)-mediated DNA damage response (DDR) pathway. Topoisomerases, such as TOP1 and TOP2B, play a role in ecDNA replication-induced DNA double-strand breaks (DSBs). A subset of these elevated DSBs persists into the mitotic phase and is primarily repaired by the alternative non-homologous end joining (alt-NHEJ) pathway, which involves POLθ and LIG3. Correspondingly, ecDNA maintenance requires DDR, and inhibiting DDR impairs the circularization of ecDNA. In summary, we demonstrate reciprocal interactions between ecDNA maintenance and DDR, providing new insights into the detection and treatment of ecDNA+ tumors.
DOI: 10.1016/j.cell.2025.04.012
Source: https://www.cell.com/cell/abstract/S0092-8674(25)00414-3
