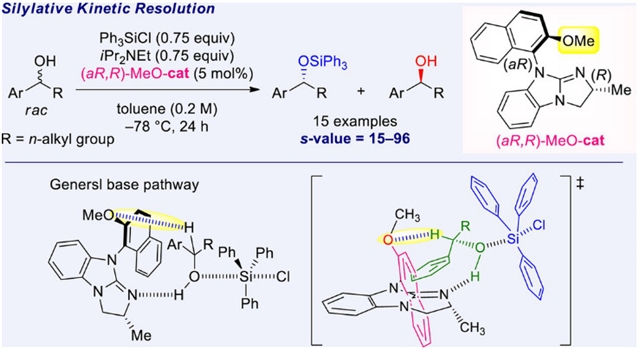
近日,日本岛根大学Kenya Nakata团队研究了含甲氧基轴向手性胍催化无环仲苯甲醇的硅烷基动力学拆分。相关论文于2025年4月23日发表在《中国化学》杂志上。
研究组合成了具有含甲氧基轴向手性的新型手性胍催化剂。随后,通过使用氯硅烷对外消旋醇进行甲硅烷基化动力学拆分,考察了它们的催化能力。基于催化剂的X射线晶体学分析,预测了甲氧基的功能作用,并描述了可能的反应途径和过渡态。还表明,催化剂上的甲氧基与底物C-1位的氢原子之间存在氢键对于获得高选择性和反应性非常重要。所提出的方法适用于各种表现出中高s值的无环芳基、杂芳基和正烷基醇= 15–96, 15个示例)。
附:英文原文
Title: Silylative Kinetic Resolution of Acyclic Secondary Benzylic Alcohols Catalyzed by Chiral Guanidine Having Axial Chirality Containing a Methoxy Group
Author: Yuki Homma, Takahisa Ikeue, Kenya Nakata
Issue&Volume: 2025-04-23
Abstract: New chiral guanidine catalysts having axial chirality containing a methoxy group were synthesized. Subsequently, their catalytic ability was examined by applying the silylative kinetic resolution of racemic alcohols using chlorosilanes. Based on an X-ray crystallographic analysis of the catalysts, the functional role of the methoxy group was predicted, and the plausible reaction pathways and a transition state were described. It was also revealed that the existence of hydrogen bonding between the methoxy group on the catalyst and hydrogen atom at C-1 position of the substrates was of great importance for attaining high selectivity and reactivity. The proposed method is applicable to various acyclic aryl, heteroaryl, and normal-alkyl alcohols exhibiting medium to high s-values (s =15–96, 15 examples).
DOI: 10.1002/cjoc.70025
Source: https://onlinelibrary.wiley.com/doi/full/10.1002/cjoc.70025
Chinese Journal of Chemistry:《中国化学》,创刊于1983年。隶属于Wiley,最新IF:5.4
官方网址:https://onlinelibrary.wiley.com/journal/16147065
投稿链接:https://mc.manuscriptcentral.com/cjoc
