近日,中国科学技术大学杨上峰团队研究了内嵌富勒烯中无配体Ni2−阴离子的镧系元素-镍分子金属间配合物。相关论文于2025年4月23日发表在《自然-化学》杂志上。
过渡金属(TM)通常表现出丰富的氧化还原化学性质,可以以各种氧化态存在。在大多数情况下,TM带正电荷。强π接受配体已被证明可以稳定处于形式负氧化态的TMs分子配合物。相比之下,不含有机配体的TM阴离子仍然很少见,仅限于基于第三行TM的金属间化合物,如金或铂。
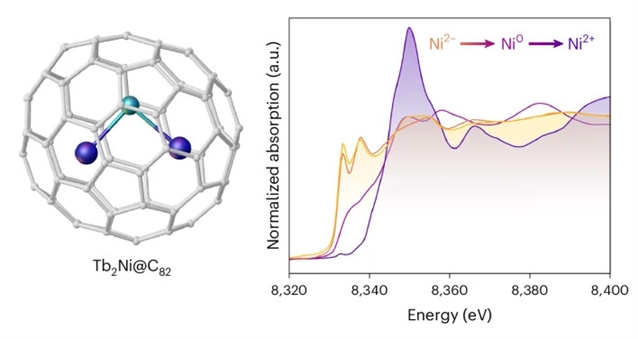
研究组报告了空气稳定的镧系元素-镍分子金属间化合物的合成,其特征是富勒烯中限制的无配体Ni2−,即,Tb2Ni@C82。带电的Tb2Ni镧系元素镍簇形成纯金属路易斯对,具有强极化的Tb-Ni共价键,键长在2.50-2.57Å范围内。X射线吸收光谱支持镍的-2氧化态,电子计数为3d104s2,与光谱和磁性测量以及理论研究一致。这一发现开辟了一种有效的方法,通过将金属间簇限制在分子碳笼内,稳定具有难以捉摸的亲核TM阴离子的金属间簇。
附:英文原文
Title: Lanthanide–nickel molecular intermetallic complexes featuring a ligand-free Ni2 anion in endohedral fullerenes
Author: Chuai, Panfeng, Hu, Ziqi, Yao, Yang-Rong, Jiang, Zhanxin, Ullah, Aman, Zhao, Ya, Cheng, Weiren, Chen, Muqing, Coronado, Eugenio, Yang, Shangfeng, Shi, Zujin
Issue&Volume: 2025-04-23
Abstract: Transition metals (TMs) typically exhibit rich redox chemistry and can be found in various oxidation states. In most cases, TMs are positively charged. Strong π-accepting ligands have been shown to stabilize molecular complexes with TMs in formal negative oxidation states. By contrast, organic-ligand-free TM anions remain rare, limited to intermetallic compounds based on third-row TMs such as gold or platinum. Here we report the synthesis of air-stable lanthanide–nickel molecular intermetallic complexes featuring a ligand-free Ni2 confined within fullerenes, namely, Tb2Ni@C82. The charged Tb2Ni lanthanide nickelide cluster forms metal-only Lewis pairs, featuring strongly polarized Tb–Ni covalent bonds with short bond lengths in the range of 2.50–2.57. X-ray absorption spectroscopy supports the 2 oxidation state of Ni with 3d104s2 electron count, in line with the spectroscopic and magnetic measurements, and theoretical study. This finding opens up an efficient way to stabilize intermetallic clusters with elusive nucleophilic TM anions by confining them inside molecular carbon cages.
DOI: 10.1038/s41557-025-01802-2
Source: https://www.nature.com/articles/s41557-025-01802-2
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
