近日,美国传染病组教授David A. Relman及其课题组揭示了短暂使用抗生素会促使人类肠道细菌产生低成本耐药性。这一研究成果发表在2025年4月23日出版的国际学术期刊《自然》上。
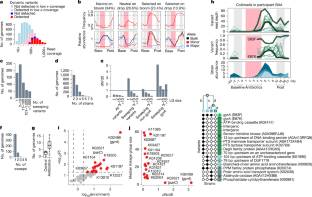
为了解释耐药性是如何在体内进化的,研究人员将60名人类参与者暴露于环丙沙星和主题纵向粪便样本中,并采用一种新的计算方法来组装参与者体内5,665个共生细菌种群的基因组。对230万个多态性序列变异的分析显示,513个种群经历了选择性扫描。研究小组发现了以DNA回转酶为中心的趋同进化,以及在其他基因组位点存在分散选择压力的证据。大约10%的易感细菌种群通过扫描进化出耐药性,其中包括在旋切酶中特定氨基酸的替换。Gyrase的进化与暴露期间相对丰度下降的大种群有关。扫描持续超过10次在大多数情况下是几周,预计一年内不会恢复。靶向扩增表明,gyrase突变在参与者体内从头产生,没有可测量的适应度成本。这些发现表明,短暂的环丙沙星暴露驱动肠道共生菌的耐药性进化,突变在暴露后很长时间内持续存在。这项研究强调了人类肠道促进耐药性进化的能力,并确定了影响细菌在体内适应的关键基因组和生态因素。
据悉,了解抗生素主题与抗菌素耐药性演变之间的关系对于有效的抗生素管理至关重要。然而,动物模型和体外实验很难复制现实世界的条件。
附:英文原文
Title: Brief antibiotic use drives human gut bacteria towards low-cost resistance
Author: Yaffe, Eitan, Dethlefsen, Les, Patankar, Arati V., Gui, Chen, Holmes, Susan, Relman, David A.
Issue&Volume: 2025-04-23
Abstract: Understanding the relationship between antibiotic use and the evolution of antimicrobial resistance is vital for effective antibiotic stewardship. Yet, animal models and in vitro experiments poorly replicate real-world conditions1. To explain how resistance evolves in vivo, we exposed 60 human participants to ciprofloxacin and used longitudinal stool samples and a new computational method to assemble the genomes of 5,665 populations of commensal bacterial species within participants. Analysis of 2.3 million polymorphic sequence variants revealed 513 populations that underwent selective sweeps. We found convergent evolution focused on DNA gyrase and evidence of dispersed selective pressure at other genomic loci. Roughly 10% of susceptible bacterial populations evolved towards resistance through sweeps that involved substitutions at a specific amino acid in gyrase. The evolution of gyrase was associated with large populations that decreased in relative abundance during exposure. Sweeps persisted for more than 10weeks in most cases and were not projected to revert within a year. Targeted amplification showed that gyrase mutations arose de novo within the participants and exhibited no measurable fitness cost. These findings revealed that brief ciprofloxacin exposure drives the evolution of resistance in gut commensals, with mutations persisting long after exposure. This study underscores the capacity of the human gut to promote the evolution of resistance and identifies key genomic and ecological factors that shape bacterial adaptation in vivo.
DOI: 10.1038/s41586-025-08781-x
Source: https://www.nature.com/articles/s41586-025-08781-x
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
