中国科学院分子植物科学卓越创新中心范敏锐研究团队报道了质体/寄生虫ATP/ADP转运体的结构与机制。2025年3月12日出版的《自然》发表了这项成果。
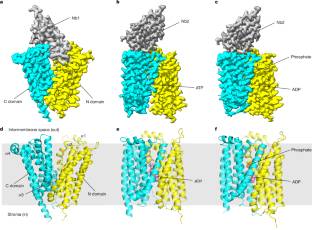
在这里,小组报告了两种质体/寄生虫类型的ATP/ADP易位子在载脂蛋白和底物结合状态下的低温电镜结构。ATP/ ADP结合口袋位于转运子N和C结构域之间的界面,口袋内保守的天冬酰胺残基对底物特异性至关重要。该转运器通过涉及作为刚体的两个域的相对旋转的摇杆开关交替存取机构操作。他们的研究结果为理解能量寄生和内共生中ATP跨膜转运提供了重要的见解,并为开发抗专性细胞内寄生虫的药物提供了结构基础。
据介绍,三磷酸腺苷(ATP)是所有活细胞的主要能量货币。代谢受损的专性细胞内寄生虫,如人类病原体沙眼衣原体和普拉兹氏立克次体,可以通过一种独特的ATP/二磷酸腺苷(ADP)转运器从宿主细胞获取ATP,该转运器介导ATP输入到寄生虫细胞中,ADP和磷酸盐输出到寄生虫细胞中,从而允许利用宿主细胞的能量储备(也称为能量寄生)。这种类型的ATP/ADP转运体也存在于原生生物的专性胞内共生体和植物和藻类的质体中,并在内共生中发挥重要作用。质体/寄生虫型ATP/ADP转运子在系统发育和功能上与线粒体ATP/ADP转运子不同,其结构和转运机制尚不清楚。
附:英文原文
Title: Structure and mechanism of the plastid/parasite ATP/ADP translocator
Author: Lin, Huajian, Huang, Jian, Li, Tianming, Li, Wenjuan, Wu, Yutong, Yang, Tianjiao, Nian, Yuwei, Lin, Xiang, Wang, Jiangqin, Wang, Ruiying, Zhao, Xiaohui, Su, Nannan, Zhang, Jinru, Wu, Xudong, Fan, Minrui
Issue&Volume: 2025-03-12
Abstract: Adenosine triphosphate (ATP) is the principal energy currency of all living cells. Metabolically impaired obligate intracellular parasites, such as the human pathogens Chlamydia trachomatis and Rickettsia prowazekii, can acquire ATP from their host cells through a unique ATP/adenosine diphosphate (ADP) translocator, which mediates the import of ATP into and the export of ADP and phosphate out of the parasite cells, thus allowing the exploitation of the energy reserves of host cells (also known as energy parasitism). This type of ATP/ADP translocator also exists in the obligate intracellular endosymbionts of protists and the plastids of plants and algae and has been implicated to play an important role in endosymbiosis. The plastid/parasite type of ATP/ADP translocator is phylogenetically and functionally distinct from the mitochondrial ATP/ADP translocator, and its structure and transport mechanism are still unknown. Here we report the cryo-electron microscopy structures of two plastid/parasite types of ATP/ADP translocators in the apo and substrate-bound states. The ATP/ADP-binding pocket is located at the interface between the N and C domains of the translocator, and a conserved asparagine residue within the pocket is critical for substrate specificity. The translocator operates through a rocker-switch alternating access mechanism involving the relative rotation of the two domains as rigid bodies. Our results provide critical insights for understanding ATP translocation across membranes in energy parasitism and endosymbiosis and offer a structural basis for developing drugs against obligate intracellular parasites.
DOI: 10.1038/s41586-025-08743-3
Source: https://www.nature.com/articles/s41586-025-08743-3
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
