新研究证明了非手性配体可以被主题化以获得具有极高构型稳定性的手性钌半夹层配合物,这一成果由上海交通大学张江高级研究所马佳佳
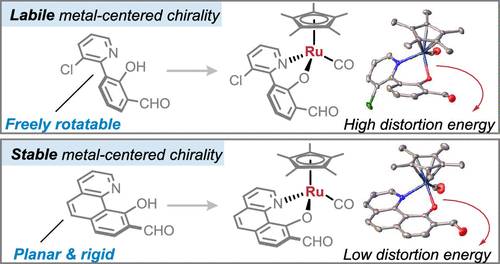
课题组人员证明了非手性配体可以被主题化以获得具有极高构型稳定性的手性钌半夹层配合物。成功的关键是引入刚性双齿配体,吡啶基和酚基之间的二面角最小,这被证明是有效的防止外消旋。计算研究揭示了能量因素对成功配体设计的刚性和平面结构所带来的特殊构型稳定性的贡献。这些具有光学活性的手性钌配合物通过nhc催化动力学拆分得到,具有优异的选择性(s因子高达200,ee高达99%)。该研究团队进一步证明了它们是一种高效的手性醛催化剂,用于甘氨酸酯和对醌的不对称1,6共轭加成。
据了解,虽然半夹层Ru(II)配合物具有以金属为中心的手性,但由于金属立体中心的构型不稳定性,利用其金属为中心的手性进行不对称合成的有效策略一直具有挑战性。以金属为中心的手性通常由对映纯配体介导,与非手性配体相比,对映纯配体占据相对狭窄的化学空间。
附:英文原文
Title: Half-Sandwich Ru(II) Complexes Featuring Metal-Centered Chirality: Configurational Stabilization by Ligand Design, Preparation via Kinetic Resolution, and Application in Asymmetric Catalysis
Author: Hui Liang, Gabriel N. Morais, Gang Chen, Wei Tang, Jing Zhao, Chuanyong Wang, K. N. Houk, Shuming Chen, Jiajia Ma
Issue&Volume: February 12, 2025
Abstract: While it is well established that half-sandwich Ru(II) complexes possess metal-centered chirality, effective strategies to leverage their metal-centered chirality toward asymmetric synthesis have been challenging due to the configurational lability of the metal stereocenters. The metal-centered chirality is typically mediated by enantiopure ligands, which occupy a relatively narrow chemical space compared to their achiral counterparts. We demonstrate that achiral ligands can be used to access chiral-at-ruthenium half-sandwich complexes with exceptionally high configurational stability. Key to success is the introduction of a rigid bidentate ligand with a minimized dihedral angle between the pyridyl and phenolic moieties, which proved effective for preventing racemization. Computational studies revealed the energetic factors contributing to the exceptional configurational stability enabled by the rigid and planar structure of the successful ligand design. These optically active chiral-at-ruthenium complexes incorporating aldehyde moieties were obtained by NHC-catalyzed kinetic resolution with excellent selectivities (s-factor up to >200, ee up to 99%). We further demonstrate that they are highly effective chiral aldehyde catalysts for the asymmetric 1,6-conjugate addition of glycine ester and para-quinone methide.
DOI: 10.1021/jacs.4c16928
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.4c16928
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
