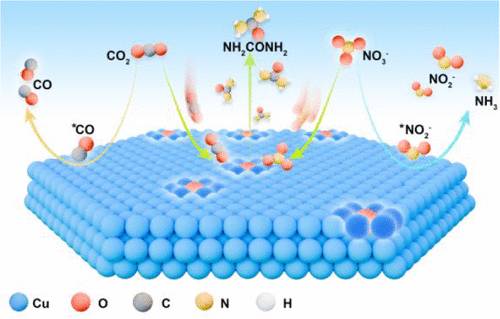
在这项研究中,研究人员证明了氧化物衍生铜纳米片(OL-Cu)中的残余晶格氧可以有效地调节电子分布,它们激活邻近的铜原子并产生缺电子铜(Cuδ+)位点。这些Cuδ+位点增强了CO2吸附,稳定了*CO中间体,使相邻Cuδ+位点能够定向吸附NO3-。该机制缩短了C-N偶联途径,在-0.7V条件下,尿素产率高达298.67mmol h-1 g-1,在~95mA cm-2的高电流密度下,平均法拉第效率为31.71%。原位光谱测量证实了Cuδ+位点的形成,并跟踪了尿素合成过程中关键中间体(即*CO, *NO, *OCNO和*NOCONO)的演变。密度泛函理论计算表明,Cuδ+位点促进了*CO和*NO3、*OCNO和*NO3的相邻共吸附,显著改善了C-N耦合动力学。该研究强调了晶格氧在促进相邻共吸附和提高C-N偶联选择性方面的关键作用。
据悉,铜基催化剂上CO2和NO3-的电化学耦合为尿素生产提供了一种可持续的策略,同时也解决了废水反硝化问题。然而,铜表面对CO2和NO3-的低效随机吸附限制了关键碳氮中间体的相互作用,从而阻碍了碳氮的有效耦合。
附:英文原文
Title: Lattice Oxygen-Driven Co-Adsorption of Carbon Dioxide and Nitrate on Copper: A Pathway to Efficient Urea Electrosynthesis
Author: Xiaoxiao Wei, Shao-Qing Liu, Hengjie Liu, Yutian Ding, Peng-Xia Lei, Shuwen Wu, Li Song, Xian-Zhu Fu, Jing-Li Luo
Issue&Volume: February 10, 2025
Abstract: The electrochemical coupling of CO2 and NO3– on copper-based catalysts presents a sustainable strategy for urea production while simultaneously addressing wastewater denitrification. However, the inefficient random adsorption of CO2 and NO3– on the copper surface limits the interaction of the key carbon and nitrogen intermediates, thereby impeding efficient C–N coupling. In this study, we demonstrate that the residual lattice oxygen in oxide-derived copper nanosheets (OL-Cu) can effectively tune the electron distribution, thus activating neighboring copper atoms and generating electron-deficient copper (Cuδ+) sites. These Cuδ+ sites enhance CO2 adsorption and stabilize *CO intermediates, which enables the directional NO3– adsorption at adjacent Cuδ+ sites. This mechanism shortens the C–N coupling pathway and achieves a urea yield of up to 298.67 mmol h–1 g–1 at 0.7V versus RHE, with an average Faradaic efficiency of 31.71% at a high current density of ~95mA cm–2. In situ spectroscopic measurements confirmed the formation of Cuδ+ sites and tracked the evolution of the key intermediates (i.e., *CO, *NO, *OCNO, and *NOCONO) during urea synthesis. Density functional theory calculations revealed that Cuδ+ sites promote adjacent coadsorption of *CO and *NO3, as well as *OCNO and *NO3, significantly improving C–N coupling kinetics. This study underscores the critical role of lattice oxygen in facilitating adjacent coadsorption and improving C–N coupling selectivity.
DOI: 10.1021/jacs.4c16801
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.4c16801
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
