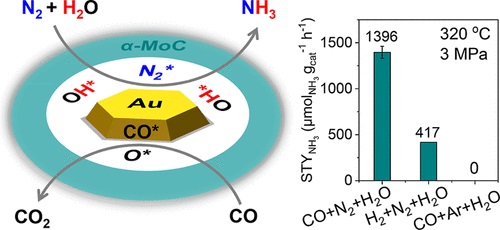
本文报道了在Au/ α-MoC1-x催化剂上,以CO为氧受体,由N2和H2O直接合成氨的工艺,从而绕过了在温和条件下N2与H2O活化的热力学限制。该工艺在低至100℃的温度下合成了NH3,在320℃下NH3的产率为1396 μmolNH3 gcat-1 h-1。研究小组发现Au/ α-MoC1-x边界提供了Auδ+物质用于CO吸附,腾出氧覆盖的Mo位点用于N2吸附和H2O解离为OH*物质,从而使N2加氢成NH3。该工艺通过氧受体和双功能Au/ α-MoC1-x的协同作用,使氮和水直接合成氨。
据了解,氮(N2)和水(H2O)直接合成氨(NH3)是一种很有前途的途径,它绕过了高能耗的制氢过程,实现了氨的节能生产,但它受到不利的反应热力学的限制。
附:英文原文
Title: Direct Ammonia Synthesis from Nitrogen and Water at Mild Conditions
Author: Baibei Zhao, Wei Hu, Chenxi Guan, Jun Mao, Yunlong Zhang, Rui Huang, Liang Yu, Dehui Deng
Issue&Volume: December 2, 2025
Abstract: Direct ammonia (NH3) synthesis from nitrogen (N2) and water (H2O) is a promising route for achieving energy-efficient NH3 production by circumventing the energy-intensive H2 production process, yet it is limited by unfavorable reaction thermodynamics. Herein, we report a direct ammonia synthesis process from N2 and H2O with CO as the oxygen acceptor to remove oxygen from H2O over a Au/α-MoC1–x catalyst, thereby bypassing the thermodynamic limitation of N2 activation with H2O under mild conditions. This process achieves NH3 synthesis at temperatures as low as 100 °C, yielding 1396 μmolNH3 gcat–1 h–1 of NH3 at 320 °C. We disclose that the Au/α-MoC1–x boundary offers Auδ+ species for CO adsorption to vacate oxygen-covered Mo sites for N2 adsorption and H2O dissociation to OH* species, enabling stepwise N2 hydrogenation to NH3. This process enables direct ammonia synthesis from nitrogen and water through the synergistic cooperation of the oxygen acceptor and bifunctional Au/α-MoC1–x.
DOI: 10.1021/jacs.5c17318
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c17318
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
