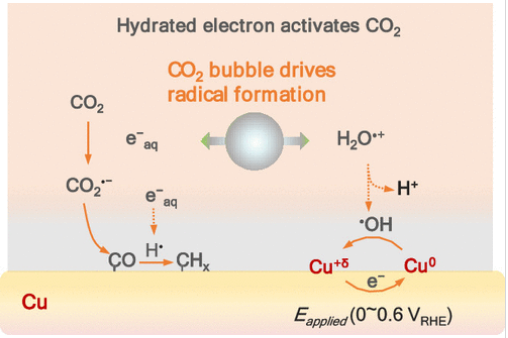
近日,电子科技大学崔春华团队报道了电化学CO2还原过程中水合电子绕过1.9 V激活势垒。这一研究成果发表在2025年12月17日出版的《美国化学会志》上。
传统电化学CO2还原被认为需要催化表面来克服惰性CO2分子的高活化能垒。
研究组发现,在碳酸氢盐溶液中,CO2微气泡界面生成的水合电子(e-aq)能够在不依赖催化剂或外加偏压的条件下直接活化CO2。通过自旋捕集质谱技术,可直接观测到CO2•–自由基中间体(CO2 + e-aq → CO2•–);而采用惰性铂电极的实验证实了后续溶液相中CO的生成(CO2•– + H+/•H → CO + H2O)。
出乎意料的是,原位拉曼光谱显示,即使在0.6 VRHE(较已报道的CO2活化电位高出约2.0 V)条件下,铜电极表面仍能观察到CO吸附与C–H键的形成。施加的还原电位(0–0.6 VRHE,高于H+/H2还原起始电位)通过氧化性自由基清除作用调控界面e-aq/•H浓度,从而实现了溶液介导的氢化过程。这些发现确立了一个前所未有的模型:电解质驱动的过程与传统的表面电催化并行发挥作用,这对“CO2活化必须依赖催化表面”的长期固有认知提出了挑战。
附:英文原文
Title: Hydrated Electrons Bypass the 1.9 V Activation Barrier in Electrochemical CO2 Reduction
Author: Di Wu, Ruijuan Zhao, Lei Li, Chunhua Cui
Issue&Volume: December 17, 2025
Abstract: Electrochemical CO2 reduction is traditionally thought to require catalytic surfaces to overcome the high activation barrier of inert CO2 molecules. Here, we demonstrate that in bicarbonate solutions, hydrated electrons (e–aq) generated at the CO2 microbubble interfaces can activate CO2 independently of catalysts or applied bias. Using spin-trapping mass spectrometry, we directly observe CO2– radical intermediates (CO2 + e–aq → CO2–), while inert Pt electrode experiments confirm subsequent solution-phase CO generation (CO2– + H+/H → CO + H2O). Unexpectedly, in situ Raman spectroscopy reveals CO adsorption and C–H bond formation on Cu even at 0.6 VRHE, approximately 2.0 V above the reported potential for CO2 activation. The applied reduction potential (0–0.6 VRHE, above the onset of the H+/H2 reduction potential) modulates the interfacial e–aq/H concentration through oxidative radical scavenging, enabling solution-mediated hydrogenation. These findings establish an unprecedented model in which electrolyte-driven processes operate in parallel with conventional surface electrocatalysis, challenging long-standing assumptions about the necessity of catalytic surfaces for CO2 activation.
DOI: 10.1021/jacs.5c15039
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c15039
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
