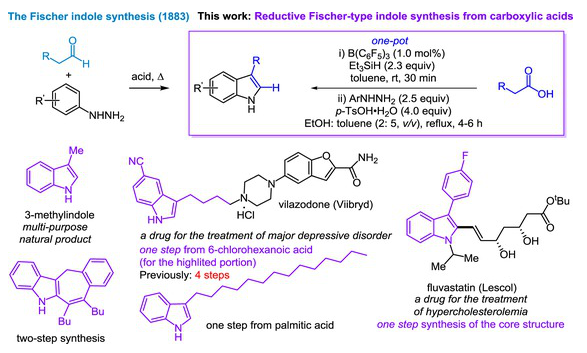
近日,厦门大学黄培强团队研究了用羧酸代替醛的还原型Fischer吲哚合成。这一研究成果于2025年12月17日发表在《中国化学》杂志上。
针对经典醛起始的Fischer吲哚合成法的若干不足,研究组报道了一种基于羧酸原料的还原型Fischer吲哚合成新策略。该方法通过一锅串联反应实现:首先在三(五氟苯基)硼催化下,羧酸与三乙基硅烷发生硅氢化反应生成相应的O,O-二硅基缩醛;随后在对甲苯磺酸介导下与芳基肼发生转胺化形成腙中间体;最终通过后期Fischer吲哚环化完成转化。在此过程中,羧酸充当了醛等价物的角色。利用该一锅法策略,研究组成功合成了一系列3-取代吲哚化合物,包括3,4-、3,5-及3,6-二取代吲哚衍生物。
该方法的实用价值通过以下实例得以凸显:以廉价易得的商品化羧酸和芳基肼为原料,高效合成了多用途天然产物3-甲基吲哚、抗抑郁药物维拉佐酮(商品名Viibryd)商业化生产的关键中间体,以及治疗高胆固醇血症常用处方药的核心骨架结构。该一锅法反应对卤素(氟、氯、溴、碘)、甲氧基、氰基、三氟甲基等多种官能团均表现出良好耐受性——这些基团既是药物与农用化学品研发的关键结构单元,也可作为进一步修饰吲哚产物的反应位点。如通过两步反应合成四环吲哚衍生物的实例所示,该方法为吲哚类化合物的结构修饰提供了灵活高效的合成平台。
附:英文原文
Title: Reductive Fischer-Type Indole Synthesis Employing Carboxylic Acids as Surrogates of Aldehydes
Author: Yan Wang, Xin Xu, Pei-Qiang Huang
Issue&Volume: 2025-12-17
Abstract: To tackle some shortcomings of the classic Fischer indole synthesis employing aldehydes as starting materials, we report herein a reductive Fischer-type indole synthesis based on carboxylic acids. This method comprises a tandem sequence involving B(C6F5)3-catalyzed hydrosilylation of carboxylic acids with Et3SiH to generate the corresponding O,O-disilyl acetals, p-TsOH-mediated transamination with arylhydrazines to form hydrazones, and a late-stage Fischer indole cyclization. In this manner, carboxylic acids act as surrogates of aldehydes. Using this one-pot protocol, a series of 3-substituted indoles, including 3,4-, 3,5-, and 3,6-disubstituted indole derivatives have been synthesized. The utility of this protocol is demonstrated by the efficient synthesis of the versatile natural product 3-methylindole, the key intermediate in the commercial production of the antidepressant drug Vilazodone (Viibryd), and the core scaffold of one of the most frequently prescribed agents for the treatment of hypercholesterolemia—all from inexpensive, commercially available carboxylic acids and arylhydrazines. The one-pot reaction tolerates several functional groups such as halogen (F, Cl, Br, I), MeO, CN, and CF3, which are either key moieties for the development of medicinal and agrochemical agents, or can be used as a handle for the further elaboration of the indole products, as demonstrated by the two-step synthesis of a tetracyclic indole derivative.
DOI: 10.1002/cjoc.70385
Source: https://onlinelibrary.wiley.com/doi/10.1002/cjoc.70385
Chinese Journal of Chemistry:《中国化学》,创刊于1983年。隶属于Wiley,最新IF:5.4
官方网址:https://onlinelibrary.wiley.com/journal/16147065
投稿链接:https://mc.manuscriptcentral.com/cjoc
