近日,日本RIKEN可持续资源科学中心(CSRS)Ryuhei Nakamura团队报道了电化学释氯过程中,阳离子的水化熵调节氯离子的扩散。2025年12月8日出版的《自然-化学》杂志发表了这项成果。
杂质离子是电解水环境多样化的主要挑战。特别是低品位水中的氯杂质,降低了水电解器的选择性和使用寿命。
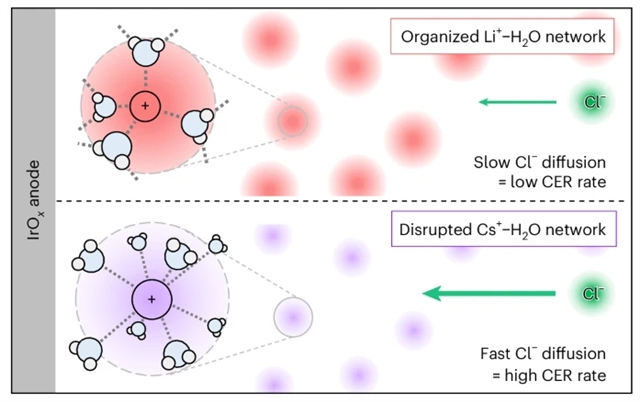
研究组证明了碱阳离子可以调节氯离子的扩散,使水氧化的反应选择性显著提高。旋转环盘电极测量在Levich图中显示出异常的正截距,表明微分势垒与阳离子相关,但与转速无关。为了使这种势垒合理化,研究组提出了对Levich模型的一个简单修改,其中阳离子相关的扩散系数比体溶液的至少低两个数量级。最大熵势和水化结构熵均表明,当第一水化壳层结构刚性(Li+ > Na+ > H+ > K+ > Cs+)时,扩散势垒增大。该发现提供了一种在与水电解相关的高电流密度下抑制杂质驱动的副反应的策略。
附:英文原文
Title: Hydration entropy of cations regulates chloride ion diffusion during electrochemical chlorine evolution
Author: Lim, Taejung, Ooka, Hideshi, Yu, Yuhang, Murakami, Takeharu, Wada, Satoshi, Nakamura, Ryuhei
Issue&Volume: 2025-12-08
Abstract: Impurity ions pose a major challenge towards diversifying water sources for electrolysis. In particular, chloride impurities in low-grade water diminish the selectivity and longevity of water electrolysers. Here we demonstrate that alkali cations can regulate chloride diffusion, allowing a marked improvement in the reaction selectivity of water oxidation. Rotating ring-disk electrode measurements exhibit anomalous positive intercepts in the Levich plot, indicating a diffusional barrier that is cation dependent yet independent of rotational speed. To rationalize this barrier, we propose a simple modification to the Levich model, in which the cation-dependent diffusion coefficient is at least two orders of magnitude lower than that of the bulk solution. The potential of maximum entropy and the structural entropy of hydration both indicate that the diffusion barrier increases when the first hydration shell is structurally rigid (Li+>Na+>H+>K+>Cs+). Our findings offer a strategy to suppress impurity-driven side reactions at the high current densities relevant to water electrolysis.
DOI: 10.1038/s41557-025-02014-4
Source: https://www.nature.com/articles/s41557-025-02014-4
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
