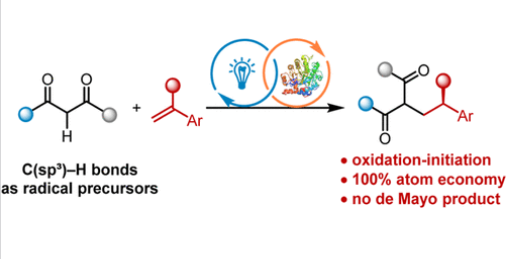
近日,上海交通大学叶俊涛团队报道了1,3-二羰基的光酶C(sp3)-H官能化实现苯乙烯的对映选择性氢烷基化。这一研究成果发表在2025年11月19日出版的《美国化学会志》上。
在过去十年中,光酶催化已发展成为实现挑战性不对称自由基转化的强效策略。尽管已取得显著进展,但该方法通常需要预官能化的自由基前体。因此,利用广泛存在的C–H键作为光酶催化的自由基前体具有重要价值。
研究组报道了通过工程化黄素依赖性烯还原酶,在中性pH条件下直接氧化常见的1,3-二羰基化合物,实现了苯乙烯的光酶催化氢烷基化反应,从C(sp3)–H键产生亲电性碳中心自由基。该策略实现了100%原子经济性,并克服了醛醇缩合和德梅奥反应等常见竞争途径,能以良好收率和优异对映选择性获得多种手性富集的1,3-二羰基产物。计算研究表明,自由基生成通过质子耦合电子转移过程进行,并揭示了对映选择性的起源。
附:英文原文
Title: Photoenzymatic C(sp3)–H Functionalization of 1,3-Dicarbonyls Enables Enantioselective Hydroalkylation of Styrenes
Author: Ermeng Wang, Qiaoyu Zhang, Qinglong Shi, Xiaoyu Wang, Ting Ma, Yixue Wu, Binju Wang, Juntao Ye
Issue&Volume: November 19, 2025
Abstract: Photoenzymatic catalysis has evolved into a powerful strategy for achieving challenging asymmetric radical transformations over the past decade. While considerable progress has been made, prefunctionalized radical precursors are generally required. Therefore, leveraging ubiquitous C–H bonds as radical precursors for photoenzymatic catalysis is highly desirable. Here we report that engineered flavin-dependent ene-reductases enable photoenzymatic hydroalkylation of styrenes via direct oxidation of abundant 1,3-dicarbonyls at neutral pH, generating electrophilic carbon-centered radicals from C(sp3)–H bonds. This strategy achieves 100% atom economy and overcomes common competing pathways such as Aldol condensation and the de Mayo reaction, affording a broad array of enantioenriched 1,3-dicarbonyl products in good yield with excellent enantioselectivity. Computational studies revealed that radical generation proceeds via a proton-coupled electron transfer process and elucidated the origin of enantioselectivity.
DOI: 10.1021/jacs.5c17564
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c17564
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
