近日,中国科学院上海有机所黄正团队研究了亲电性Co(II)卡宾在还原环丙烷反应中的ECEC机理。2025年11月17日,《美国化学会杂志》发表了这一成果。
金属碳烯一直被认为是有机金属化学中的关键反应中间体。近年来,宝石二氯烷烃的催化还原工艺已成为烯烃环丙烷制备非稳定给体碳的一种很有前途的方法。然而,由于金属碳中间体固有的不稳定性,催化循环的研究仍然具有挑战性。
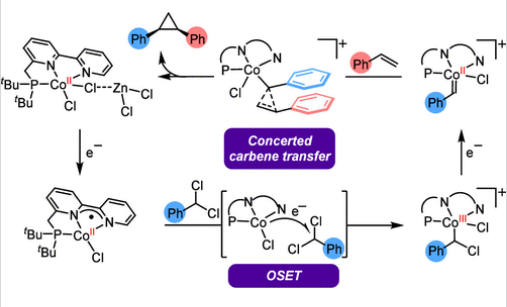
研究组通过结合电分析技术与密度泛函理论(DFT)计算实验-计算联用方法,阐明了(PNN)Co催化剂(PNN = 联吡啶膦)催化烯烃还原环丙烷化反应遵循ECEC机理。该过程始于ZnCl2辅助下对(PNN)CoIICl2 (1)的单电子还原,生成具有还原性π-自由基阴离子特征的[(PNN)•–]CoIICl (2)。通过哈米特线性自由能关系分析确定,2对芳基偕二氯甲烷的活化经由外层电子转移过程,生成阳离子苄基Co(III)物种[(PNN)CoIIICl(苄基)]+ZnCl3– (A)。在首次EC步骤后,循环伏安研究、控制电位电解及反应性对比实验共同证实了第二次EC过程涉及将A单电子还原为阳离子Co(II)卡宾物种[(PNN)CoIICl(卡宾)]+ZnCl3– (C),该中间体能有效进行后续卡宾转移反应。哈米特分析与计算数据共同支持将中间体C归类为亲电性Co(II)卡宾物种,合理解释了顺式选择性烯烃环丙烷化反应遵循协同非自由基加成路径。该研究构建了多电子多步反应机理框架,对开发使用廉价金属的可持续催化转化具有重要价值。
附:英文原文
Title: An ECEC Mechanism via an Electrophilic Co(II) Carbene in Reductive Cyclopropanation
Author: Bin Cao, Hao-Dong Tan, Guixia Liu, Xuebing Leng, Xiao-Song Xue, Zheng Huang
Issue&Volume: November 17, 2025
Abstract: Metal carbenes have long been recognized as a pivotal reactive intermediate in organometallic chemistry. Recently catalytic reductive processes with gem-dichloroalkanes have emerged as a promising strategy to generate nonstabilized donor carbenes for alkene cyclopropanation. However, the catalytic cycle remains challenging to study largely due to the inherent instability of metal carbene intermediates. In this study, we elucidated an ECEC mechanism for the (PNN)Co-catalyzed (PNN = bipyridyl phosphine) reductive cyclopropanation of alkenes through an integrated experimental-computational approach combining electroanalytical techniques and density functional theory (DFT) calculations. Initiated by ZnCl2-assisted one-electron reduction of (PNN)CoIICl2 (1) to [(PNN)–]CoIICl (2) featuring a reduced π-radical anion, activation of aryl gem-dichloromethanes by 2 proceeds via outer-sphere electron transfer (OSET) as determined by Hammett linear free-energy relationship (LFER) analysis, producing a cationic benzyl Co(III) species [(PNN)CoIIICl(benzyl)]+ZnCl3– (A). Following the first EC, cyclic voltammetry studies, controlled potential electrolysis, and reactivity comparison experiments collectively established the second EC process involving one-electron reduction of A to a cationic Co(II) carbene [(PNN)CoIICl(carbene)]+ZnCl3– (C) that is effective for the following carbene transfer. LFER analysis and computational data support the assignment of intermediate C as an electrophilic Co(II) carbene, rationalizing a concerted, nonradical addition pathway for cis-selective alkene cyclopropanation. This work provides a multielectron, multistep mechanistic framework, which could be valuable for developing sustainable catalytic transformations that employ base metals.
DOI: 10.1021/jacs.5c13356
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c13356
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000