近日,哈尔滨工业大学(深圳)夏吾炯团队报道了化学选择性电解控制芳烃脱芳烃合成1,4-氢烷基化和C(sp2)-H烷基化。2025年11月17日,《自然-化学》杂志发表了这一成果。
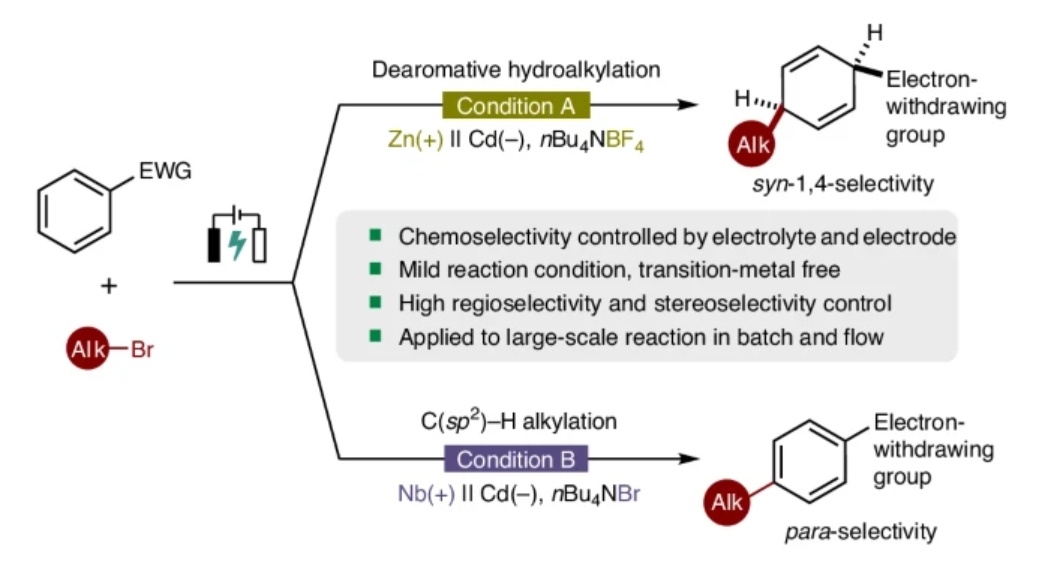
脱芳构官能团化作为一种高效的合成策略,能够快速构建复杂化学结构。该过程面临的核心挑战在于如何克服芳环固有的芳香性。研究组利用有机电解的潜力,开发了针对缺电子芳烃与杂芳烃的脱芳构顺式-1,4-氢烷基化反应。这种电化学方法在温和、操作简便且可放大的条件下进行,能够以高化学选择性、区域选择性和立体选择性合成烷基化顺式-1,4-环己二烯。
值得注意的是,该烷基化策略具有可控性与可切换性:当以铌板为阳极、四丁基溴化铵为支撑电解质时,可通过电解实现(杂)芳烃的对位选择性C(sp2)–H烷基化。两类反应均展现出广泛的底物适用性,并与多种缺电子芳烃及溴代烷烃呈现优良的兼容性。此外,研究组通过初步机理研究与密度泛函理论计算,阐明了反应机理,并对观测到的化学选择性、区域选择性和立体选择性给出了合理解释。
附:英文原文
Title: Dearomative syn-1,4-hydroalkylation and C(sp2)-H alkylation of arenes controlled by chemoselective electrolysis
Author: Wan, Chao, Yang, Chao, Rueping, Magnus, Zhu, Chen, Guo, Lin, Xia, Wujiong
Issue&Volume: 2025-11-17
Abstract: Dearomative functionalization of arenes represents a powerful synthetic strategy for the rapid assembly of complex chemical architectures. A significant challenge in this process is overcoming the inherent aromaticity of arenes. Here, leveraging the potential of organic electrolysis, we show the development of a dearomative syn-1,4-hydroalkylation reaction targeting electron-deficient arenes and heteroarenes. This electrochemical approach, conducted under mild, operationally straightforward and scalable conditions, facilitates the synthesis of alkylated syn-1,4-cyclohexadienes with high chemoselectivity, regioselectivity and stereoselectivity. In addition, this alkylation protocol is controllable and switchable. By employing a niobium plate as the anode and nBu4NBr as the supporting electrolyte, our method enables the para-selective C(sp2)–H alkylation of (hetero)arenes via electrolysis. Both reactions exhibit broad substrate scope and demonstrate excellent compatibility with various electron-deficient arenes and alkyl bromides. Furthermore, preliminary mechanistic studies and density functional theory calculations have been performed to elucidate the reaction mechanism and to rationalize the observed chemoselectivity, regioselectivity and stereoselectivity.
DOI: 10.1038/s41557-025-02001-9
Source: https://www.nature.com/articles/s41557-025-02001-9
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
