格莱斯顿-加州大学旧金山分校基因组免疫学研究所Alexander Marson团队在研究中取得进展。他们的论文提出了FOXP3的表达依赖于细胞类型特异性顺式调控元件和转录因子通路。相关论文于2025年11月13日发表于国际顶尖学术期刊《免疫学》杂志上。
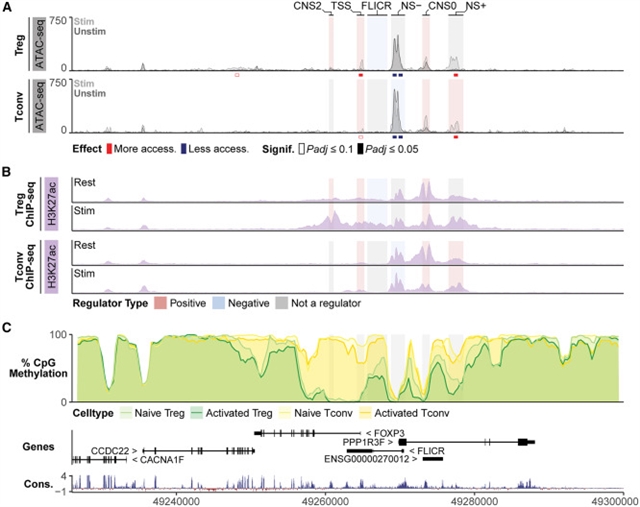
研究小组在人类Treg和Tconv细胞中对FOXP3位点和TFs进行了CRISPR筛选,以鉴定FOXP3的顺式调控元件(CREs)和反式调控元件。Tconv细胞FOXP3的表达依赖于Treg细胞CREs的一个子集,以及Tconv细胞选择性阳性(NS+)和阴性(NS)CREs。Tconv细胞CREs的组合沉默揭示了它们的上位逻辑。这些CREs被研究团队确定为FOXP3调节因子的TF占据和调节。最后,小鼠NSCRE揭示了其在Treg细胞中限制FOXP3表达的重要性。研究人员绘制了CRE和TF电路,以揭示FOXP3表达的不同细胞和物种特异性调控。
据介绍,FOXP3是一种免疫抑制调节性T细胞(Treg细胞)的谱系定义转录因子(TF)。虽然小鼠只在Treg细胞中表达FOXP3,但受刺激的常规CD4+ T细胞(Tconv细胞)在人体内也会短暂表达FOXP3。控制这些不同表达模式的机制需要阐明。
附:英文原文
Title: FOXP3 expression depends on cell-type-specific cis-regulatory elements and transcription factor circuitry
Author: Jennifer M. Umhoefer, Maya M. Arce, Sivakanthan Kasinathan, Sean Whalen, Rama Dajani, Sanjana Subramanya, Laine Goudy, Julia A. Belk, Royce Zhou, Minh T.N. Pham, Wenxi Zhang, Rosmely Hernandez, Carinna Tran, Nikhita Kirthivasan, Jacob W. Freimer, Cody T. Mowery, Vinh Nguyen, Mineto Ota, Benjamin G. Gowen, Dimitre R. Simeonov, Gemma L. Curie, Zhongmei Li, Andy Y. Chen, Jacob E. Corn, Howard Y. Chang, Qizhi Tang, Luke A. Gilbert, Ansuman T. Satpathy, Katherine S. Pollard, Alexander Marson
Issue&Volume: 2025-11-13
Abstract: FOXP3 is a lineage-defining transcription factor (TF) for immune-suppressive regulatory T cells (Treg cells). Although mice exclusively express FOXP3 in Treg cells, stimulated conventional CD4+ T cells (Tconv cells) also transiently express FOXP3 in humans. Mechanisms governing these distinct expression patterns need elucidation. Here, we performed CRISPR screens tiling the FOXP3 locus and targeting TFs in human Treg and Tconv cells to identify cis-regulatory elements (CREs) and trans-regulators of FOXP3. Tconv cell FOXP3 expression depended on a subset of Treg cell CREs, as well as Tconv-cell-selective positive (NS+) and negative (NS) CREs. Combinatorial silencing of Tconv cell CREs revealed their epistatic logic. These CREs are occupied and regulated by TFs that we identified as FOXP3 regulators. Finally, mutagenesis of murine NS CRE revealed its essentiality for restricting FOXP3 expression to Treg cells. We map CRE and TF circuitry to reveal distinct cell- and species-specific regulation of FOXP3 expression.
DOI: 10.1016/j.immuni.2025.10.020
Source: https://www.cell.com/immunity/abstract/S1074-7613(25)00474-1
Immunity:《免疫》,创刊于1994年。隶属于细胞出版社,最新IF:43.474
官方网址:https://www.cell.com/immunity/home
投稿链接:https://www.editorialmanager.com/immunity/default.aspx
