中山大学高益军研究组揭示了Paneth样转化驱动结直肠癌中KRAS和EGFR双靶向的耐药性。该项研究成果发表在2025年11月13日出版的《癌细胞》上。
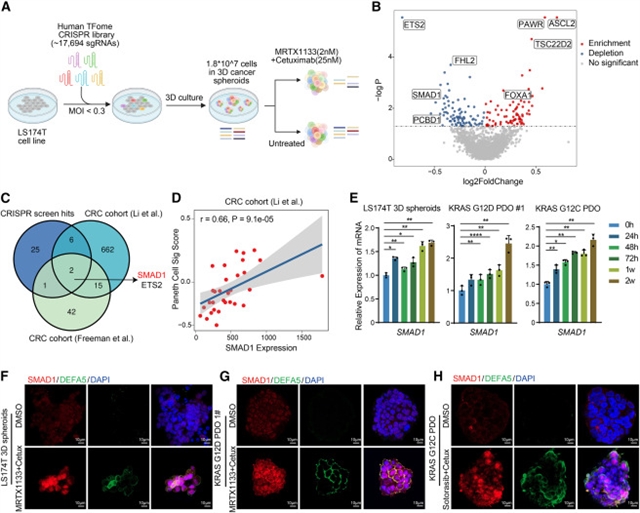
通过基因工程motheme模型、患者来源的类器官和异种移植物以及临床标本,课题组发现KRAS和EGFR联合抑制后存活的结直肠肿瘤获得了Paneth样细胞状态——一种通常局限于肠隐窝的分泌谱系。谱系追踪显示CRC细胞通过转变为Paneth样状态来逃避双重治疗。通过整合转录组学分析和CRISPR基因筛选,课题组确定SMAD1是该谱系可塑性的关键调节因子,通过直接激活FGFR3来促进抗性。FGFR3的遗传或药理学抑制可阻止Paneth样转变,恢复药物敏感性,并在多个临床前模型中与KRAS-EGFR抑制协同作用。这些研究结果表明,SMAD1-FGFR3轴触发Paneth样可塑性以驱动CRC中KRAS-EGFR双药耐药,并强调FGFR3阻断是克服可塑性驱动的药物耐受性的有希望的策略。
据了解,虽然双重KRAS和表皮生长因子受体(EGFR)抑制在治疗KRAS突变型结直肠癌(CRC)中显示出希望,但耐药性仍然是主要挑战。
附:英文原文
Title: Paneth-like transition drives resistance to dual targeting of KRAS and EGFR in colorectal cancer
Author: Yuetong Zhang, Jiaying Chen, Yong She, Zhaoyuan Fang, Yaxin Zhang, Danyun Ruan, Wenjun Guo, Jianping Liao, Weiping Zhou, Jianpei Lao, Weicheng Fang, Xingyan Pan, Wenfei Kang, Zifeng Wang, Yuanzhong Wu, Rong Deng, Lin Tian, Liqin Wang, Huilin Huang, Jian Zheng, Yan Yan, Hezhe Lu, Ruiping Wang, Rona Yaeger, Qi Zhao, Wenting Liao, Feng Wang, Yijun Gao
Issue&Volume: 2025-11-13
Abstract: While dual KRAS and epidermal growth factor receptor (EGFR) inhibition shows promise in treating KRAS-mutant colorectal cancer (CRC), resistance remains a major challenge. Using genetically engineered mouse models, patient-derived organoids and xenografts, as well as clinical specimens, we discover that colorectal tumors surviving combined KRAS and EGFR inhibition acquire a Paneth-like cell state—a secretory lineage typically confined to the intestinal crypt. Lineage tracing reveals that CRC cells evade dual therapy by transitioning into a Paneth-like state. Through integrated transcriptomic analysis and CRISPR genetic screening, we identify SMAD1 as a key regulator of this lineage plasticity, promoting resistance by directly activating FGFR3. Genetic or pharmacological inhibition of FGFR3 prevents the Paneth-like transition, restores drug sensitivity, and synergizes with KRAS-EGFR inhibition across multiple preclinical models. These findings reveal that the SMAD1-FGFR3 axis triggers Paneth-like plasticity to drive KRAS-EGFR dual therapy resistance in CRC and highlight FGFR3 blockade as a promising strategy to overcome plasticity-driven drug tolerance.
DOI: 10.1016/j.ccell.2025.10.010
Source: https://www.cell.com/cancer-cell/abstract/S1535-6108(25)00451-9
Cancer Cell:《癌细胞》,创刊于2002年。隶属于细胞出版社,最新IF:38.585
官方网址:https://www.cell.com/cancer-cell/home
投稿链接:https://www.editorialmanager.com/cancer-cell/default.aspx
