近日,中国科学技术大学龚流柱团队实现了钯催化的内部烯烃的烯丙基C-H烷基化反应。相关论文于2025年11月11日发表在《美国化学会志》上。
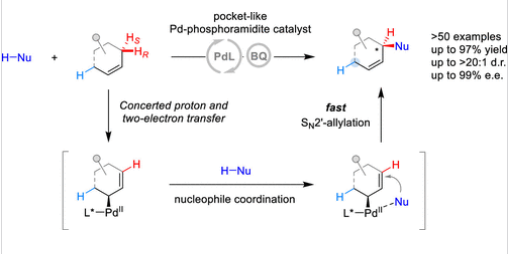
对普遍存在的内烯烃中烯丙基C–H键进行位点和对映选择性转化是不对称合成中的重大挑战。引入类似酶的手性微环境是实现烯丙基C–H键pro-R/S和位点区分的关键策略,从而避免产生复杂的区域和立体异构混合物。
研究组报道了一种通过口袋状手性磷酰胺–钯催化体系实现的高选择性烯丙基C–H键烷基化方法,适用于环状和非环状内烯烃的广泛底物范围。色散作用被证实是稳定对映区分过渡态的关键因素。在由大位阻磷酰胺配体片段界定的开放口袋中,电子和空间性质相似的烯丙基C–H键仍能被有效区分。该过程最终生成的手性σ-烯丙基钯中间体可快速参与SN2′-烯丙基化反应,精确地将官能团安装在最初被活化手性氢的对应位置。
附:英文原文
Title: Palladium-Catalyzed Site– and Enantiodifferentiating Allylic C–H Alkylation of Internal Alkenes
Author: Zhi-Peng Shi, Zi-Han Lin, Ye-Xuan Xu, Long-Fei Fan, Pu-Sheng Wang, Xin Hong, Liu-Zhu Gong
Issue&Volume: November 11, 2025
Abstract: The site- and enantioselective transformation of allylic C–H bonds in ubiquitous internal alkenes represents a significant challenge in asymmetric synthesis. Introducing an enzyme-like chiral environment is a crucial strategy to achieve pro-R/S and site-differentiation of allylic C–H bonds, thereby circumventing the formation of complex regio- and stereoisomeric mixtures. In this work, we report a highly site- and enantiodifferentiating allylic C–H alkylation of internal alkenes through pocket-like chiral phosphoramidite-Pd catalysis. This method is effective for a wide spectrum of cyclic and acyclic internal alkenes. Dispersion interactions are highlighted as playing a pivotal role in stabilizing the enantiodifferentiating transition states of allylic C–H bond cleavage. Within an open pocket delineated by the segments of bulky phosphoramidite ligand, electronically and sterically similar allylic C–H bonds are discriminated. This process culminates in the generation of chiral σ-allylpalladium intermediates that rapidly engage in SN2′-allylation, installing the functional group precisely at the position of the initially cleaved prochiral hydrogen.
DOI: 10.1021/jacs.5c13527
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c13527
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
