2025年10月29日出版的《自然》杂志发表了罗切斯特大学Vera Gorbunova团队的最新成果,他们的研究开发出了长寿弓头鲸DNA修复改善的证据。
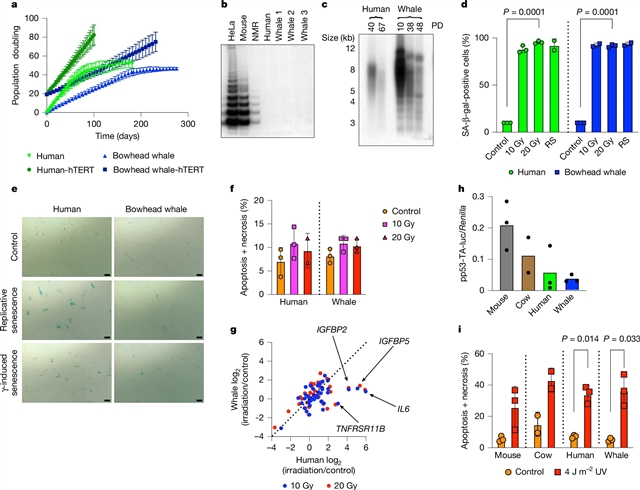
为了了解弓头鲸耐癌的机制,该团队研究了鲸原代成纤维细胞恶性转化所需的致癌击中数。出乎意料的是,弓头鲸成纤维细胞发生恶性转化所需的致癌撞击比人类成纤维细胞要少。
然而,弓头鲸细胞表现出比其他哺乳动物细胞更强的DNA双链断裂修复能力和保真度,以及更低的突变率。该团队发现冷诱导RNA结合蛋白CIRBP在弓头鲸成纤维细胞和组织中高度表达。弓头鲸CIRBP增强了人类细胞非同源端连接和同源端重组修复,减少了微核形成,促进了DNA端保护,并刺激了体外末端连接。果蝇中CIRBP的过表达延长了果蝇的寿命并提高了对辐照的抵抗力。这些发现为以下假设提供了支持证据:弓头鲸不是依靠额外的肿瘤抑制基因来预防肿瘤发生,而是通过增强DNA修复来维持基因组完整性。这种策略不会消除受损细胞,而是忠实地修复它们,这可能是弓头鲸异常长寿和癌症发病率低的原因。
据悉,弓头鲸的最长寿命超过200岁,超过了所有其他哺乳动物。弓头鲸也是地球上第二大的动物,体重超过80000公斤。尽管弓头鲸的细胞数量非常多,寿命也很长,但它并不容易患癌症,这种不协调的现象被称为皮托悖论。
附:英文原文
Title: Evidence for improved DNA repair in long-lived bowhead whale
Author: Firsanov, Denis, Zacher, Max, Tian, Xiao, Sformo, Todd L., Zhao, Yang, Tombline, Gregory, Lu, J. Yuyang, Zheng, Zhizhong, Perelli, Luigi, Gurreri, Enrico, Zhang, Li, Guo, Jing, Korotkov, Anatoly, Volobaev, Valentin, Biashad, Seyed Ali, Zhang, Zhihui, Heid, Johanna, Maslov, Alexander Y., Sun, Shixiang, Wu, Zhuoer, Gigas, Jonathan, Hillpot, Eric C., Martinez, John C., Lee, Minseon, Williams, Alyssa, Gilman, Abbey, Hamilton, Nicholas, Strelkova, Ekaterina, Haseljic, Ena, Patel, Avnee, Straight, Maggie E., Miller, Nalani, Ablaeva, Julia, Tam, Lok Ming, Couderc, Chlo, Hoopmann, Michael R., Moritz, Robert L., Fujii, Shingo, Pelletier, Amandine, Hayman, Dan J., Liu, Hongrui, Cai, Yuxuan, Leung, Anthony K. L., Zhang, Zhengdong, Nelson, C. Bradley, Abegglen, Lisa M., Schiffman, Joshua D., Gladyshev, Vadim N., Maley, Carlo C., Modesti, Mauro, Genovese, Giannicola, Simons, Mirre J. P., Vijg, Jan, Seluanov, Andrei, Gorbunova, Vera
Issue&Volume: 2025-10-29
Abstract: At more than 200 years, the maximum lifespan of the bowhead whale exceeds that of all other mammals. The bowhead is also the second-largest animal on Earth1, reaching over 80,000kg. Despite its very large number of cells and long lifespan, the bowhead is not highly cancer-prone, an incongruity termed Peto’s paradox2. Here, to understand the mechanisms that underlie the cancer resistance of the bowhead whale, we examined the number of oncogenic hits required for malignant transformation of whale primary fibroblasts. Unexpectedly, bowhead whale fibroblasts required fewer oncogenic hits to undergo malignant transformation than human fibroblasts. However, bowhead whale cells exhibited enhanced DNA double-strand break repair capacity and fidelity, and lower mutation rates than cells of other mammals. We found the cold-inducible RNA-binding protein CIRBP to be highly expressed in bowhead fibroblasts and tissues. Bowhead whale CIRBP enhanced both non-homologous end joining and homologous recombination repair in human cells, reduced micronuclei formation, promoted DNA end protection, and stimulated end joining in vitro. CIRBP overexpression in Drosophila extended lifespan and improved resistance to irradiation. These findings provide evidence supporting the hypothesis that, rather than relying on additional tumour suppressor genes to prevent oncogenesis3,4,5, the bowhead whale maintains genome integrity through enhanced DNA repair. This strategy, which does not eliminate damaged cells but faithfully repairs them, may be contributing to the exceptional longevity and low cancer incidence in the bowhead whale.
DOI: 10.1038/s41586-025-09694-5
Source: https://www.nature.com/articles/s41586-025-09694-5
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
