近日,中国医学科学院/北京协和医学院付海根团队设计了一种亚胺还原酶用于对映选择性合成轴手性二聚体酰胺。相关论文于2025年10月27日发表在《美国化学会志》上。
轴手性酰胺化合物因芳环-酰胺键的旋转受限而产生独特的轴向手性,在生物活性分子和不对称催化领域具有广泛应用。然而,目前催化不对称合成该类化合物的方法仍不成熟,且尚未见生物催化途径的报道。
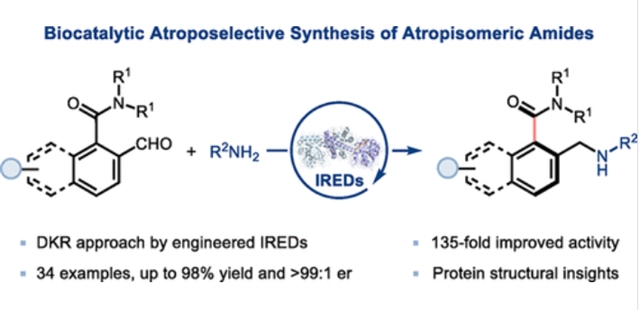
研究组首次报道通过工程化亚胺还原酶(IREDs)实现动态动力学拆分,从而高效合成轴手性酰胺的生物催化策略。基于结构指导对源自白色库兹涅尔菌的IRED进行改造,获得了能够催化多种萘甲酰胺类与苯甲酰胺类化合物立体汇聚合成的四重突变体(IRED-68-M4),其反应收率优异且对映选择性极高(最高达98%收率,>99:1 er)。研究组还成功实现了轴向手性萘甲酰胺的克级规模合成。通过蛋白质X射线晶体学与分子模拟研究,进一步揭示了IRED-68-M4突变体催化性能提升的结构基础。
附:英文原文
Title: Engineering an Imine Reductase for Enantioselective Synthesis of Atropisomeric Amides
Author: Zhouchang Yao, Runze Meng, Zitian Zhou, Luyao Yu, Zhiyun Wu, Longqing Tang, Tianzhang Qiao, Ke Li, Ling Huang, Danqing Song, Haigen Fu
Issue&Volume: October 27, 2025
Abstract: Atropisomeric amides possess unique axial chirality arising from the rotation-restricted Caryl–Camide bond and find broad application in bioactive molecules and asymmetric catalysis. However, catalytic asymmetric methods for their synthesis remain underdeveloped, with no biocatalytic approaches reported. Herein, we report the first efficient biocatalytic strategy for the atroposelective synthesis of atropisomeric amides via dynamic kinetic resolution using engineered imine reductases (IREDs). Structure-guided engineering of an IRED from Kutzneria albida provided a quadruple mutant (IRED-68-M4) capable of catalyzing the stereoconvergent synthesis of diverse napthamides and benzamides in high yields and excellent enantioselectivities (up to 98% yield, >99:1 er). Gram-scale synthesis of an axially chiral napthamide was also demonstrated. Moreover, protein X-ray crystallography and molecular modeling studies revealed the structural basis of the enhanced catalytic performance of the IRED-68-M4 variant.
DOI: 10.1021/jacs.5c12724
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c12724
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
