近日,南京大学
手性独立基金属配合物的发展一直受到低效合成方法的阻碍,明显落后于成熟的Cp金属配合物。
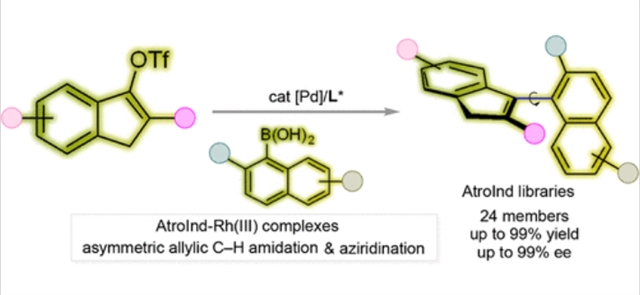
在这项研究中,课题组通过不对称钯催化的Suzuki-Miyaura交叉偶联反应,提出了一种流线型高效合成新型AtroInds的方法。利用这种方法,可以很容易地构建包含不同空间和电子性质的AtroInd库。所得的AtroInd-Rh (III)配合物制备简单,并进行了全面的表征,在不对称催化中表现出优异的催化效率。计算研究为反应途径和控制对映体选择性的关键因素提供了深刻的见解,从而增强了对其机理的理解。这种综合方法不仅解决了目前手性茚的合成挑战,而且通过先进的合成方法为新型配体库的设计和合成建立了新的范例。
附:英文原文
Title: Atropisomeric Indene (AroInd) Libraries: Design, Catalytic Synthesis, and Applications
Author: Yuan Zheng, Jingran Zhang, Jingyi Bai, Minyan Wang, Zhuangzhi Shi
Issue&Volume: October 27, 2025
Abstract: The development of chiral indenyl metal complexes has been hindered by inefficient synthetic methods, significantly lagging behind the well-established Cp metal complexes. In this study, we present a streamlined and highly efficient synthesis of novel atropisomeric indenes (AtroInds) via asymmetric palladium-catalyzed Suzuki–Miyaura cross-coupling reactions. Leveraging this method, AtroInd libraries, encompassing varying steric and electronic properties, were readily constructed. The resulting AtroInd–Rh(III) complexes were prepared with ease and subjected to comprehensive characterization, revealing exceptional catalytic efficiency in asymmetric catalysis. Computational studies provided profound insights into the reaction pathway and the critical factors governing enantioselectivity, thereby enhancing our mechanistic understanding. This integrated approach not only addresses the prevailing synthetic challenges in the preparation of chiral indenes but also establishes a new paradigm for the design and synthesis of novel ligand libraries through advanced synthetic methodologies.
DOI: 10.1021/jacs.5c13866
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c13866
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
