近日,北京科技大学张跃团队实现了操纵界面电荷分布以减少水分。这一研究成果于2025年10月15日发表在《美国化学会志》上。
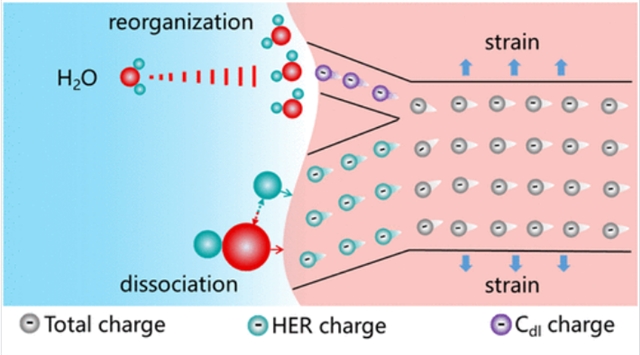
多相电催化过程通常涉及电极/电解质界面处非法拉第过程与法拉第过程的复杂相互作用,其反应动力学往往呈现对外加偏压的指数依赖关系。然而,偏压的不可分割特性阻碍了对各过程的独立认知与精准调控。通过单一介质协同调控包含多反应步骤的这两个过程,仍是亟待攻克的重要难题。
研究组提出通过精确操控界面电荷分布来解决这一关键问题。通过在NiCo2S4中实施晶格应变工程,研究组在碱性析氢反应(HER)中实现了非法拉第电荷与法拉第电荷的最优匹配。这不仅缓解了静电势对水分子重组过程的限制,同时增强了化学势对水分子解离过程的积极贡献。此外,研究证实适中的eg轨道占据数是法拉第电荷显著增加的根本原因,二者呈现火山型关系。这种界面电荷重分布策略构建了多步反应动力学的协同优化路径,可进一步拓展至析氢反应之外的多相电催化反应体系。
附:英文原文
Title: Manipulating Interfacial Charge Distribution for Water Reduction
Author: Jing Wu, Xin Wang, Wenhao Zheng, Yu Sun, Yong Xie, Zhen Tian, Wenjia Zhang, Guowei He, Zhuojian Liang, Zhuo Kang, Yue Zhang
Issue&Volume: October 15, 2025
Abstract: Heterogeneous electrocatalysis often involves a complex interplay of the non-Faradaic process and the Faradaic process at the electrode/electrolyte interface, with the reaction kinetics typically exhibiting an exponential dependence on applied bias. However, the indivisible nature of bias hinders independent understanding and fine-tuning of each process. Synergistically regulating these two processes containing multiple reaction steps via one single, yet unified mediator remains a formidable challenge. Here, precise manipulation of the interfacial charge distribution is proposed to tackle this critical challenge. Through lattice strain engineering in NiCo2S4, the optimal amount matching between non-Faradaic charges and Faradaic charges is yielded during alkaline hydrogen evolution reaction (HER). As a result, the restriction of electrostatic potential on the water molecule reorganization process is alleviated, and the positive contribution of chemical potential to the water molecule dissociation process is concurrently strengthened. Furthermore, the moderate eg orbital filling is evidenced as the essential origin of a substantial increase in Faraday charges, the relationship between which is in the volcanic form. This interfacial charge redistribution enabled a synergistic optimization route of kinetic multisteps that can be further extended to other heterogeneous electrocatalytic reactions beyond HER.
DOI: 10.1021/jacs.5c09493
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c09493
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
