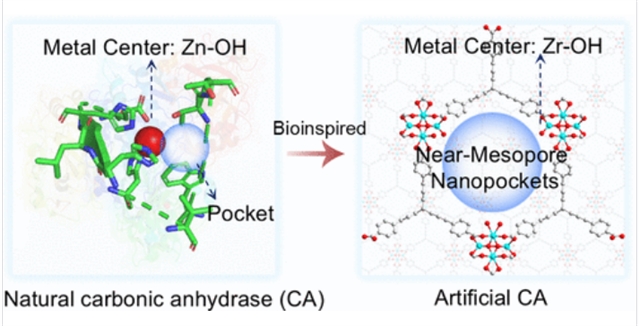
碳酸酐酶(CA)模拟酶在二氧化碳相关的催化应用中显示出很大的潜力,而目前设计的CA模拟酶仅限于模拟活性位点和纳米孔。
研究组报道了通过Zr有机框架结构重新设计的仿生Zr-OH位点和尺寸可调的纳米孔,用于有效催化CO2水化和HCO3-脱水。竞争配位调制提高了Zr-OH位点密度和可及性。配体的高连通性增加了Zr-有机骨架对配体缺陷的敏感性,而其固有的大尺寸提供了接近介孔尺度的纳米孔。该设计具有优异的4-硝基苯醋酸酯水解性能(Vmax = 3.86 μM s-1, TON = 29.92 × 10-3 s-1),优于最先进的CA模拟物。
同时,研究组系统地阐明了Zr-OH的催化机理,并表明它与CO2相互作用形成双齿碳酸盐中间体──类似于天然CA的催化机理。此外,Zr-有机骨架还能催化碳酸氢盐脱水,这符合天然CA的双向特性。这项工作为模拟酶中原子位点的设计和纳米孔的调控提供了基本的见解,也为制备用于二氧化碳相关应用的高性能CA模拟物提供了有前景的策略。
附:英文原文
Title: Bioinspired Carbonic Anhydrase Mimics with Zr–OH Sites and Size-Tunable Nanopockets for Efficient Bidirectional Catalysis of CO2 Hydration
Author: Wenjie Xu, Chao He, Qian Zhu, Shuang Li, Hao Wu, Yi Wang, Mao Wang, Shudong Sun, Chong Cheng, Changsheng Zhao
Issue&Volume: October 14, 2025
Abstract: Carbonic anhydrase (CA)-mimetic enzymes display promising potential in CO2-related catalytic applications, whereas currently designed CA mimics are limited to mimicking the active site and nanopockets. Here, we report the de novo design of bioinspired Zr–OH sites and size-tunable nanopockets via Zr-organic framework structures for the efficient catalysis of CO2 hydration and HCO3– dehydration. Competitive coordination modulation enhances Zr–OH site density and accessibility. The high connectivity of the ligand increases the sensitivity of the Zr-organic framework to ligand defects, while its inherently large size provides near-mesoporous-scale nanopockets. This design enables exceptional 4-nitrophenyl acetate hydrolysis performance (Vmax = 3.86 μM s–1 and TON = 29.92 × 10–3 s–1), superior to the state-of-the-art CA mimics. Meanwhile, we systematically elucidate the catalytic mechanism of Zr–OH and show that it interacts with CO2 to form bidentate carbonate intermediates─similar to the catalytic mechanism of natural CA. Furthermore, the Zr-organic framework can also catalyze the dehydration of bicarbonate, which is consistent with the bidirectional character of natural CA. This work provides fundamental insights into the design of atomic sites and the regulation of nanopockets in enzyme mimics, as well as a promising strategy for preparing high-performance CA mimics for CO2-related applications.
DOI: 10.1021/jacs.5c13405
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.5c13405
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
