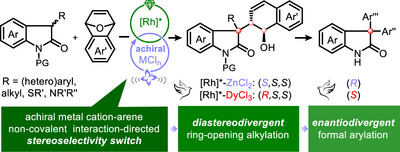
近日,云南大学邵志会团队报道了手性催化剂诱导的四元立体中心立体选择性转换:恶吲哚的开环烷基化和对映体芳基化。2025年10月13日,《德国应用化学》杂志发表了这一成果。
制定统一策略来实现非对映散和对映散是一个突出但具有挑战性的目标。
研究组提出了一种非手性路易斯酸定向立体发散的新策略。采用非手性金属盐作为控制元件,实现非对映选择性开关。通过简单地将非手性路易斯酸从非镧系d嵌段金属盐(ZnCl2)转变为镧系f嵌段金属盐(DyCl3),实现了外消旋氧吲哚与杂环烯烃的非对映发散不对称开环烷基化反应,得到了一系列具有三个连续立体中心的3,3-二取代氧吲哚,具有优异的对映选择性(高达>99% ee)和非对映选择性(高达>95:5 dr)。
接下来,通过简单地切换非手性金属路易斯酸助催化剂,同时保持相同的手性催化剂,实现了对映发散的形式芳化,得到了具有三芳基取代的季立体中心的手性3,3-二芳基氧吲哚。手性3,3-吲哚的不同转化传递了合成的主题化合物。这些手性3,3-二取代吲哚对HCT116结直肠癌细胞显示出显著的抗增殖活性。这些化合物的相对构型和绝对构型对其生物活性有显著影响,进一步突出了催化不对称立体发散合成的重要性。
附:英文原文
Title: Achiral Catalyst-Induced Stereoselectivity Switch at Quaternary Stereocenter: Diastereodivergent Ring-Opening Alkylation and Enantiodivergent Arylation of Oxindoles
Author: Jinhai Huang, Hui Xu, Yucheng Pang, Lin Liu, Xuanchen Wan, Wei Wang, Lifei Gan, Jiangtao Ren, Fangzhi Peng, Yanfeng Dang, Yingqing Ou, Yu-Hua Deng, Zhihui Shao
Issue&Volume: 2025-10-13
Abstract: The development of a unified strategy to achieve both diastereodivergence and enantiodivergence is a prominent yet challenging objective. In this study, a new strategy involving achiral Lewis acid -directed stereodivergence has been developed. An achiral metal salt is used as a control element to achieve a diastereoselectivity switch, which is unusual. By simply changing the achiral Lewis acids from a non-lanthanide d-block metal salt (ZnCl2) to a lanthanide f-block metal salt (DyCl3), a diastereodivergent asymmetric ring-opening alkylation of racemic oxindoles with oxabicyclic alkenes has been achieved, leading to a new series of chiral 3,3-disubstituted oxindoles with three contiguous stereocenters bearing a dihydronaphthalen-1-ol motif with excellent enantioselectivities (up to > 99% ee) and diastereoselectivities (up to > 95:5 dr). Next, by simply switching the achiral metal Lewis acid cocatalysts while maintaining the same chiral catalyst, an enantiodivergent formal arylation has been achieved, providing chiral 3,3-diaryl oxindoles featuring a triaryl-substituted quaternary stereocenter. Diverse transformations of the chiral 3,3-oxindoles delivered synthetically useful compounds. Those chiral 3,3-disubstituted oxindoles have shown significant antiproliferative activity against HCT116 colorectal cancer cells. The relative and absolute configurations of these compounds exert prominent effects on the bioactivities, further highlighting the remarkable importance of catalytic asymmetric stereodivergent synthesis.
DOI: 10.1002/anie.202507941
Source: https://onlinelibrary.wiley.com/doi/10.1002/anie.202507941
Angewandte Chemie:《德国应用化学》,创刊于1887年。隶属于德国化学会,最新IF:16.823
官方网址:https://onlinelibrary.wiley.com/journal/15213773
投稿链接:https://www.editorialmanager.com/anie/default.aspx
