近日,美国麻省理工学院Surendranath, Yogesh团队报道了热化学杂解加氢催化通过极化驱动氢化物转移进行。该研究于2025年10月9日发表在《自然-化学》杂志上。
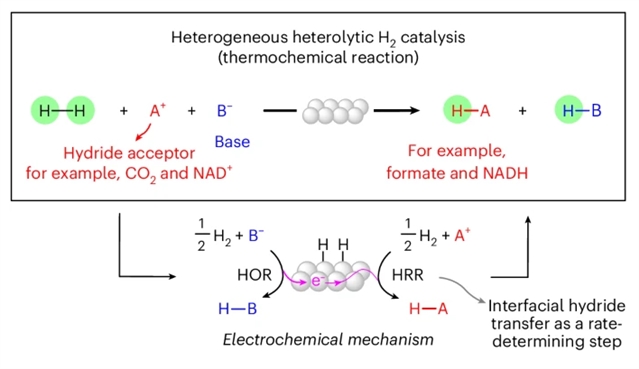
异多元氢化反应将H2裂解为氢化物受体和质子受体,构成了跨越化学价值链的关键反应类别,包括二氧化碳加氢生成甲酸和从烟酰胺腺嘌呤二核苷酸(NAD+)再生NADH。这些反应的主要机理模型援引经典的表面反应步骤,在很大程度上忽略了界面电荷分离的作用。
研究组量化了催化剂在转换过程中的电化学电位,并发现了支持这类整体热化学反应的界面电化学氢化物转移机制的证据。研究组发现质子受体诱导金属催化剂表面的自发电化学极化,从而控制表面M-H中间体的热力学水力,并驱动决速步的电化学氢化物向氢化物受体底物转移。该机制框架适用于不同的反应介质以及二氧化碳加氢生成甲酸和NAD+生成NADH,可以确定内在反应动力学,并为未来可持续加氢反应性的发展提供设计原则。
附:英文原文
Title: Thermochemical heterolytic hydrogenation catalysis proceeds through polarization-driven hydride transfer
Author: Wang, Hai-Xu, Surendranath, Yogesh
Issue&Volume: 2025-10-09
Abstract: Heterolytic hydrogenations, which split H2 across a hydride acceptor and proton acceptor, comprise a key reaction class that spans the chemical value chain, including CO2 hydrogenation to formate and NADH regeneration from nicotinamide adenine dinucleotide (NAD+). The dominant mechanistic models for heterogeneous catalysis of these reactions invoke classical surface reaction steps, largely ignoring the role of interfacial charge separation. Here we quantify the electrochemical potential of the catalyst during turnover and uncover evidence supporting an interfacial electrochemical hydride transfer mechanism for this overall thermochemical reaction class. We find that the proton acceptor induces spontaneous electrochemical polarization of the metal catalyst surface, thereby controlling the thermodynamic hydricity of the surface M–H intermediates and driving rate-determining electrochemical hydride transfer to the hydride acceptor substrate. This mechanistic framework, which applies across diverse reaction media and for the hydrogenation of CO2 to formate and NAD+ to NADH, enables the determination of intrinsic reaction kinetics and exposes design principles for the future development of sustainable hydrogenation reactivity.
DOI: 10.1038/s41557-025-01939-0
Source: https://www.nature.com/articles/s41557-025-01939-0
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
