近日,美国芝加哥大学Dong, Guangbin团队报道了使用1,2-氧杂硼环化合物作为多功能分子平台的核心多样化。这一研究成果发表在2025年10月9日出版的《自然-化学》杂志上。
在药物发现过程中,通常需要将先导化合物的核心结构改变为各种其他环系统,这通常需要重新合成单个类似物。
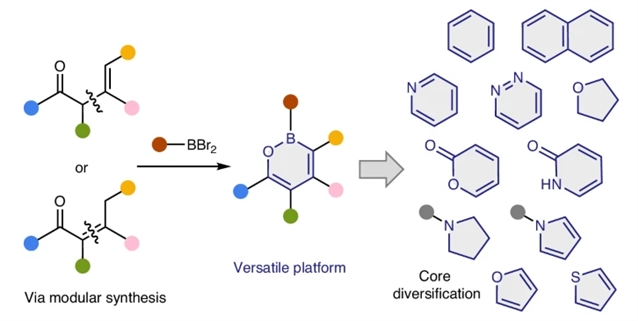
研究组报道了一种概念上全新的合成策略:以1,2-氧杂硼环化合物作为多功能分子平台,通过其共同中间体快速构建多样化的核心分子骨架。他们开发出一种温和的烯醇化/6π电环化策略,能够从易得的烯酮或烯醛高效合成1,2-氧杂硼环化合物。利用该类化合物多重反应特性,可进一步实施C-H官能团化反应,将其转化为各类芳烃、杂芳烃及非芳香性杂环化合物。最终,基于1,2-氧杂硼环的核心结构多样化策略,研究组成功实现了含有立普妥取代基但具有不同芳香环骨架的系列类似物的后期构建。
附:英文原文
Title: Core diversification using 1,2-oxaborines as a versatile molecular platform
Author: Ge, Yao, Zhu, Qi, Zhu, Yongqi, Dong, Guangbin
Issue&Volume: 2025-10-09
Abstract: In drug discovery processes, changing the core structures of lead compounds to a variety of other ring systems is often needed, which typically requires laborious de novo syntheses of individual analogues. Here we report a conceptually different approach that allows rapid access to diverse core structures from a common intermediate using 1,2-oxaborines as a versatile molecular platform. A soft enolization/6π-electrocyclization strategy has been developed to efficiently synthesize 1,2-oxaborines from readily available enones or enals. Taking advantage of their multifaceted reactivities, 1,2-oxaborines can undergo further CH functionalization and be transformed into a diverse range of arenes, heteroarenes and non-aromatic heterocycles. Finally, late-stage preparations of a suite of analogues that contain Lipitor substituents but with different aromatic cores are demonstrated using the 1,2-oxaborine-based core diversification strategy.
DOI: 10.1038/s41557-025-01971-0
Source: https://www.nature.com/articles/s41557-025-01971-0
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
