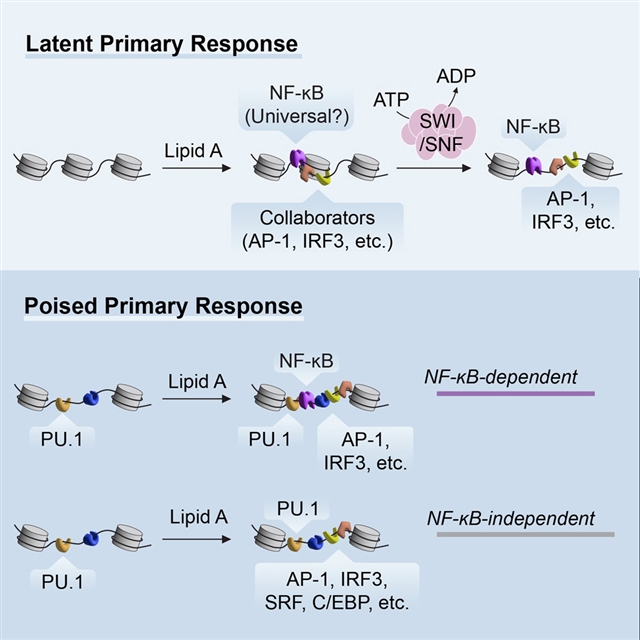
2024年3月1日出版的《免疫》杂志发表了美国科学家的一项最新研究成果。来自加州大学的Stephen T. Smale研究组的最新研究揭示,转录因子NF-κB在Toll样受体4信号的初级响应期间协调核小体重塑。
课题组研究人员旨在确定促进TLR诱导重塑的转录因子的身份。基于ATAC-seq和单细胞ATAC-seq的分析策略,富集了最有可能发生重塑的基因组区域,揭示了转录因子核因子κB(NF-κB)结合到,所有标记在TLR4配体脂多糖A(lipid A)初级响应期间重塑的高置信度峰上。
NF-κB亚基RelA和c-Rel的缺失导致高置信度ATAC-seq峰的重构丧失,并且对NF-κB结合位点进行CRISPR-Cas9突变会影响重塑。在特定区域的重塑选择性是通过与其他诱导因子的协作实现的,包括IRF3和MAP激酶诱导的因子。因此,NF-κB在其广泛贡献于可诱导的核小体重塑方面与其他TLR4激活的转录因子不同,同时具有激活组装成开放染色质的平衡增强子和启动子的能力。
据介绍,在巨噬细胞中,数百个潜在增强子和几个启动子发生可诱导的核小体重塑,塑造了对Toll样受体4(TLR4)信号的转录反应。
附:英文原文
Title: The transcription factor NF-κB orchestrates nucleosome remodeling during the primary response to Toll-like receptor 4 signaling
Author: An-Chieh Feng, Brandon J. Thomas, Prabhat K. Purbey, Filipe Menegatti de Melo, Xin Liu, Allison E. Daly, Fei Sun, Jerry Hung-Hao Lo, Lijing Cheng, Michael F. Carey, Philip O. Scumpia, Stephen T. Smale
Issue&Volume: 2024-03-01
Abstract: Inducible nucleosome remodeling at hundreds of latent enhancers and several promoters shapes the transcriptional response to Toll-like receptor 4 (TLR4) signaling in macrophages. We aimed to define the identities of the transcription factors that promote TLR-induced remodeling. An analysis strategy based on ATAC-seq and single-cell ATAC-seq that enriched for genomic regions most likely to undergo remodeling revealed that the transcription factor nuclear factor κB (NF-κB) bound to all high-confidence peaks marking remodeling during the primary response to the TLR4 ligand, lipid A. Deletion of NF-κB subunits RelA and c-Rel resulted in the loss of remodeling at high-confidence ATAC-seq peaks, and CRISPR-Cas9 mutagenesis of NF-κB-binding motifs impaired remodeling. Remodeling selectivity at defined regions was conferred by collaboration with other inducible factors, including IRF3- and MAP-kinase-induced factors. Thus, NF-κB is unique among TLR4-activated transcription factors in its broad contribution to inducible nucleosome remodeling, alongside its ability to activate poised enhancers and promoters assembled into open chromatin.
DOI: 10.1016/j.immuni.2024.02.004
Source: https://www.cell.com/immunity/fulltext/S1074-7613(24)00080-3
Immunity:《免疫》,创刊于1994年。隶属于细胞出版社,最新IF:43.474
官方网址:https://www.cell.com/immunity/home
投稿链接:https://www.editorialmanager.com/immunity/default.aspx
