可充电水系电池由于其高安全性和低成本而成为大规模储能的潜在系统。然而,开发具有高可持续性、可负担性和可逆性的水系电池是紧迫和具有挑战性的。
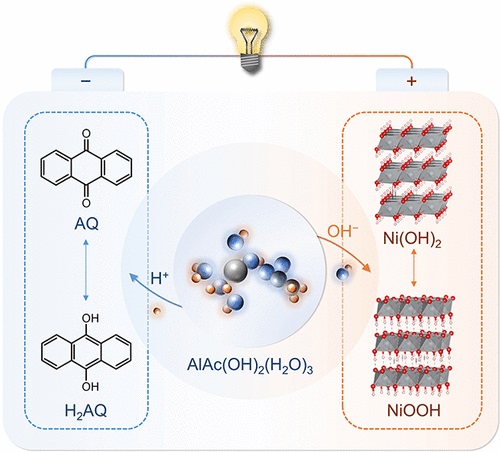
该文中,研究人员报道了一种两性羟基乙酸铝(AlAc(OH)2)电解质,它具有H+和OH–的双极电离能力,有助于蒽醌(AQ)阳极和氢氧化镍(Ni(OH)2)阴极的氧化还原反应。AlAc(OH)2(H2O)3溶剂化结构的双极电离能力源于Al3+和OH–的强极化能力。解离常数为5.0/3.0的H+/OH–解离能力强于水(14.0),这促进了电极同时稳定的氧化还原反应。
具体而言,H+吸收防止AQ阳极形成离子键,抑制电极溶解,而OH–为Ni(OH)2阴极的稳定转化反应提供了局部碱性环境。设计的AQ||Ni(OH)2电池中的AQ阳极具有243.9 mAh g–1的放电容量和300次循环后78.2%的容量保持率,具有高可逆性。此外,组装了放电容量为0.90 Ah的袋状电池,基于电池的总质量,其能量密度为44.7 Wh kg–1。
该项工作显著拓宽了水系电池的类型,代表了双极电解质的设计理念以及与H+和OH–的不同电化学反应。
附:英文原文
Title: Sustainable Aqueous Batteries Based on Bipolar Dissociation of Aluminum Hydroxyacetate Electrolyte
Author: Qiu Zhang, Xiaomeng Liu, Yong Lu, Youxuan Ni, Weiwei Xie, Zhenhua Yan, Fujun Li, Jun Chen
Issue&Volume: February 17, 2024
Abstract: Rechargeable aqueous batteries are potential systems for large-scale energy storage due to their high safety and low cost. However, developing aqueous batteries with high sustainability, affordability, and reversibility is urgent and challenging. Here we report an amphoteric aluminum hydroxyacetate (AlAc(OH)2) electrolyte with the ability of bipolar ionization of H+ and OH–, which facilitates the redox reactions at both the anthraquinone (AQ) anode and nickel hydroxide (Ni(OH)2) cathode. The bipolar ionization ability of the AlAc(OH)2(H2O)3 solvation structure results from the strong polarization ability of Al3+ and OH–. The H+/OH– dissociation ability with a dissociation constant of 5.0/3.0 is stronger than that of water (14.0), which boosts the simultaneous stable redox reactions of electrodes. Specifically, H+ uptake prevents the AQ anode from the formation of an ionic bond, suppressing the electrode dissolution, whereas OH– provides the local alkaline environment for the stable conversion reaction of the Ni(OH)2 cathode. The AQ anode in the designed AQ||Ni(OH)2 battery delivers a discharge capacity of 243.9 mAh g–1 and a capacity retention of 78.2% after 300 cycles with high reversibility. Moreover, a pouch cell with a discharge capacity of 0.90 Ah was assembled, exhibiting an energy density of 44.7 Wh kg–1 based on the total mass of the battery. This work significantly widens the types of aqueous batteries and represents a design philosophy of bipolar electrolytes and distinct electrochemical reactions with H+ and OH–.
DOI: 10.1021/jacs.3c13963
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.3c13963
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
