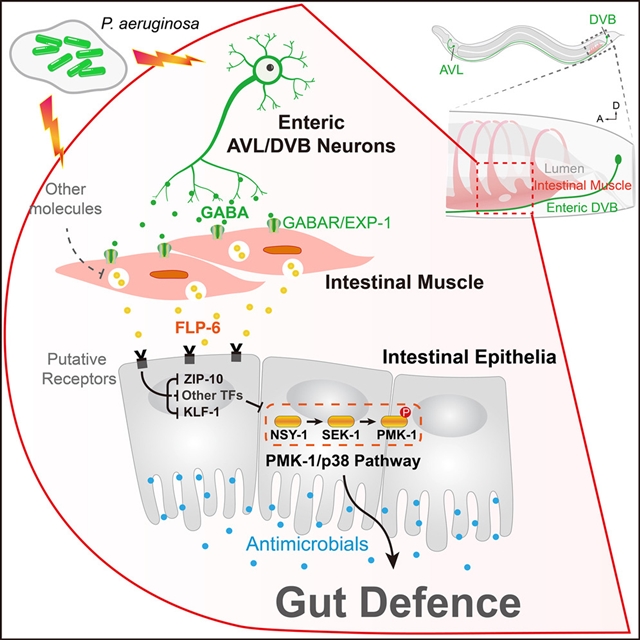
湖南大学Haijun Tu团队近期取得重要工作进展,他们研究发现,肠道神经元和肠道平滑肌之间的GABA信号传导促进秀丽隐杆线虫的先天免疫和肠道防御。相关研究成果2023年7月11日在线发表于《免疫》杂志上。
据介绍,神经系统对肠道稳态和功能至关重要,但其对肠道免疫防御的影响仍存在疑问。
通过筛选秀丽隐杆线虫的主要神经递质,研究人员发现,γ-氨基丁酸(GABA)缺乏增强了对致病性铜绿假单胞菌PA14的易感性。肠道神经元和肠平滑肌之间的GABA能信号传导以PMK-1/p38依赖性但IIS/DAF-16-和DBL-1/TGF-β非依赖性的途径促进肠道防御。转录组学分析显示,神经肽FLP-6在肠GABA能信号传导的下游发挥作用。进一步的数据表明,FLP-6由肠平滑肌细胞表达和分泌,并在肠上皮上作为旁分泌分子发挥作用。FLP-6抑制了平行工作的转录因子ZIP-10和KLF-1,并在肠上皮中汇聚到PMK-1/p38通路,用于先天免疫和肠道防御。
总之,这些发现揭示了肠道神经元-肌肉-上皮轴可能在高等生物体中演化保守。
附:英文原文
Title: GABAergic signaling between enteric neurons and intestinal smooth muscle promotes innate immunity and gut defense in Caenorhabditis elegans
Author: Junqiang Liu, Pei Zhang, Zhongfan Zheng, Muhammad Irfan Afridi, Shan Zhang, Zhiqing Wan, Xiumei Zhang, Lukas Stingelin, Yirong Wang, Haijun Tu
Issue&Volume: 2023/07/11
Abstract: The nervous system is critical for intestinal homeostasis and function, but questionsremain regarding its impact on gut immune defense. By screening the major neurotransmittersof C. elegans, we found that γ-aminobutyric acid (GABA) deficiency enhanced susceptibility to pathogenicPseudomonas aeruginosa PA14 infection. GABAergic signaling between enteric neurons and intestinal smoothmuscle promoted gut defense in a PMK-1/p38-dependent, but IIS/DAF-16- and DBL-1/TGF-β-independent,pathway. Transcriptomic profiling revealed that the neuropeptide, FLP-6, acted downstreamof enteric GABAergic signaling. Further data determined that FLP-6 was expressed andsecreted by intestinal smooth muscle cells and functioned as a paracrine moleculeon the intestinal epithelium. FLP-6 suppressed the transcription factors ZIP-10 andKLF-1 that worked in parallel and converged to the PMK-1/p38 pathway in the intestinalepithelia for innate immunity and gut defense. Collectively, these findings uncoveran enteric neuron-muscle-epithelium axis that may be evolutionarily conserved in higherorganisms.
DOI: 10.1016/j.immuni.2023.06.004
Source: https://www.cell.com/immunity/fulltext/S1074-7613(23)00261-3
Immunity:《免疫》,创刊于1994年。隶属于细胞出版社,最新IF:43.474
官方网址:https://www.cell.com/immunity/home
投稿链接:https://www.editorialmanager.com/immunity/default.aspx
