美国德克萨斯大学Ronald A. DePinho课题组发现,组蛋白去甲基化酶KDM5D上调驱动结肠癌的性别差异。相关论文于2023年6月21日在线发表在《自然》杂志上。
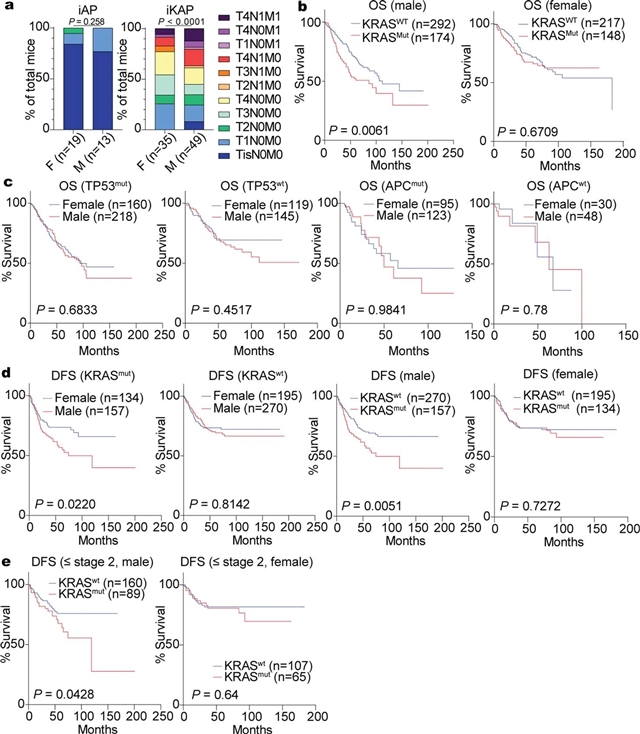
研究人员利用一个小鼠结直肠癌(CRC)模型,其通过诱导性转基因编码致癌突变体KRASG12D和Apc和Trp53肿瘤抑制因子的条件性空等位基因(命名为iKAP),发现致癌突变体KRAS(KRAS*)CRC的雄性转移率更高,结果更差。综合跨物种的分子和转录组分析发现Y染色体基因组蛋白去甲基化酶KDM5D是一个由KRAS*介导的STAT4转录因子激活驱动的转录上调基因。KDM5D依赖的染色质标记和转录组变化显示出对上皮细胞紧密连接和主要组织相容性复合体I类复合体成分调节因子的抑制。iKAP癌细胞中Kdm5d的缺失增加了紧密连接的完整性,降低了细胞的侵袭性,并增强了CD8+T细胞对癌细胞的杀伤力。相反,用Kdm5d转基因设计的iAP小鼠,专门在iAP癌细胞中提供Kdm5d的组成性表达,显示出体内肿瘤侵袭性增强的倾向。因此,KRAS*-STAT4介导的Y染色体KDM5D的上调通过破坏癌细胞粘附特性和肿瘤免疫力,对KRAS*CRC的性别差异做出了重大贡献,并为患KRAS*CRC的男性提供了一种可操作的治疗策略来降低转移风险。
据介绍,性别对癌症的发病率、谱系和结果有深刻的影响,但这种性别差异的分子和遗传基础并不明确,而且被认为是由X染色体基因和性激素造成的。这种性别差异在CRC中尤为突出,男性的转移率和死亡率更高。
附:英文原文
Title: Histone demethylase KDM5D upregulation drives sex differences in colon cancer
Author: Li, Jiexi, Lan, Zhengdao, Liao, Wenting, Horner, James W., Xu, Xueping, Liu, Jielin, Yoshihama, Yohei, Jiang, Shan, Shim, Hong Seok, Slotnik, Max, LaBella, Kyle A., Wu, Chang-Jiun, Dunner, Kenneth, Hsu, Wen-Hao, Lee, Rumi, Khanduri, Isha, Terranova, Christopher, Akdemir, Kadir, Chakravarti, Deepavali, Shang, Xiaoying, Spring, Denise J., Wang, Y. Alan, DePinho, Ronald A.
Issue&Volume: 2023-06-21
Abstract: Sex exerts a profound impact on cancer incidence, spectrum and outcomes, yet the molecular and genetic bases of such sex differences are ill-defined and presumptively ascribed to X-chromosome genes and sex hormones1. Such sex differences are particularly prominent in colorectal cancer (CRC) in which men experience higher metastases and mortality. A murine CRC model, engineered with an inducible transgene encoding oncogenic mutant KRASG12D and conditional null alleles of Apc and Trp53 tumour suppressors (designated iKAP)2, revealed higher metastases and worse outcomes specifically in males with oncogenic mutant KRAS (KRAS*) CRC. Integrated cross-species molecular and transcriptomic analyses identified Y-chromosome gene histone demethylase KDM5D as a transcriptionally upregulated gene driven by KRAS*-mediated activation of the STAT4 transcription factor. KDM5D-dependent chromatin mark and transcriptome changes showed repression of regulators of the epithelial cell tight junction and major histocompatibility complex class I complex components. Deletion of Kdm5d in iKAP cancer cells increased tight junction integrity, decreased cell invasiveness and enhanced cancer cell killing by CD8+ T cells. Conversely, iAP mice engineered with a Kdm5d transgene to provide constitutive Kdm5d expression specifically in iAP cancer cells showed an increased propensity for more invasive tumours in vivo. Thus, KRAS*-STAT4-mediated upregulation of Y chromosome KDM5D contributes substantially to the sex differences in KRAS* CRC by means of its disruption of cancer cell adhesion properties and tumour immunity, providing an actionable therapeutic strategy for metastasis risk reduction for men afflicted with KRAS* CRC.
DOI: 10.1038/s41586-023-06254-7
Source: https://www.nature.com/articles/s41586-023-06254-7
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
