日本筑波大学Kojima, Takahiko团队报道了水介质中分子铁催化剂实现甲烷选择性氧化。相关研究成果发表在2023年4月5日出版的《自然》。
使用天然气作为化学原料需要有效氧化组成烷烃,主要是甲烷。目前的工业工艺使用高温高压下的蒸汽重整来产生气体混合物,然后将其进一步转化为甲醇等产品。分子Pt催化剂也被用于将甲烷转化为甲醇,但由于过度氧化,它们的选择性通常较低——初始氧化产物往往比甲烷本身更容易氧化。
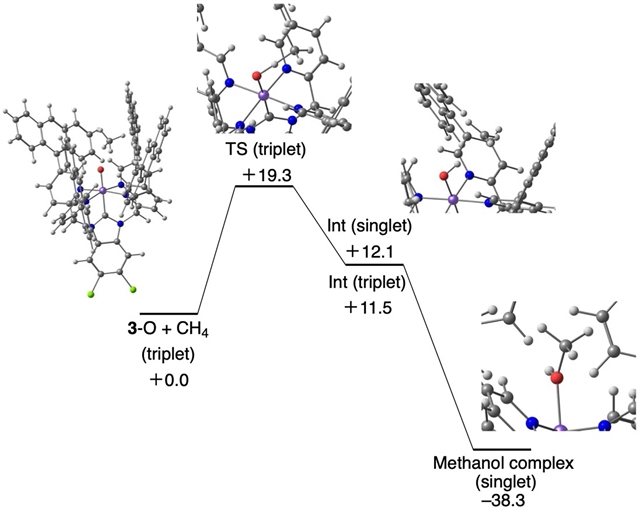
该文中,研究人员发现N-杂环卡宾连接的FeII配合物与疏水腔从水溶液中捕获疏水性甲烷底物,并在被Fe中心氧化后,将亲水性甲醇产物释放回溶液中。研究发现,增加疏水腔的尺寸可以增强这种效果,在3小时的甲烷氧化反应中,转化数为5.0×102,甲醇选择性为83%。如果能够克服在水性介质中处理甲烷所产生的运输限制,这种捕获和释放策略为利用天然丰富的烷烃资源提供了一种有效和选择性的方法。
附:英文原文
Title: Selective methane oxidation by molecular iron catalysts in aqueous medium
Author: Fujisaki, Hiroto, Ishizuka, Tomoya, Kotani, Hiroaki, Shiota, Yoshihito, Yoshizawa, Kazunari, Kojima, Takahiko
Issue&Volume: 2023-04-05
Abstract: Using natural gas as chemical feedstock requires efficient oxidation of the constituent alkanes—and primarily methane1,2. The current industrial process uses steam reforming at high temperatures and pressures3,4 to generate a gas mixture that is then further converted into products such as methanol. Molecular Pt catalysts5,6,7 have also been used to convert methane to methanol8, but their selectivity is generally low owing to overoxidation—the initial oxidation products tend to be easier to oxidize than methane itself. Here we show that N-heterocyclic carbene-ligated FeII complexes with a hydrophobic cavity capture hydrophobic methane substrate from an aqueous solution and, after oxidation by the Fe centre, release a hydrophilic methanol product back into the solution. We find that increasing the size of the hydrophobic cavities enhances this effect, giving a turnover number of 5.0×102 and a methanol selectivity of 83% during a 3-h methane oxidation reaction. If the transport limitations arising from the processing of methane in an aqueous medium can be overcome, this catch-and-release strategy provides an efficient and selective approach to using naturally abundant alkane resources.
DOI: 10.1038/s41586-023-05821-2
Source: https://www.nature.com/articles/s41586-023-05821-2
官方网址:http://www.nature.com/
