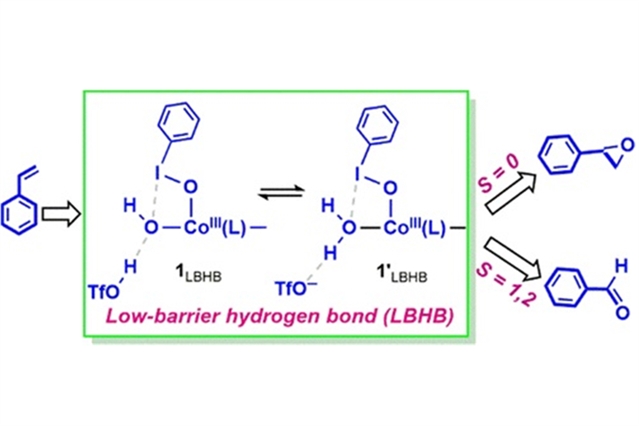
研究人员通过使用密度泛函理论计算,全面研究了三氟酸(HOTf)存在和不存在时,钴(III)-碘基苯配合物[(TQA)CoIII(OIPh)(OH)]2+ (1, TQA = 三(2-喹啉甲基)胺)氧化苯乙烯的过程。结果首次揭示了HOTf与1的羟基配体之间存在低垒氢键(LBHB),形成[(TQA)CoIII(OIPh)(HO-—HOTf)]2+ (1LBHB)和[(TQA)CoIII(OIPh)(H2O—OTf-)]2+(1’LBHB)两种价态共振结构。由于氧壁的存在,这些配合物(1LBHB和1’LBHB)不能转化为高价钴氧基。相反,苯乙烯被这些氧化剂(1LBHB和1’LBHB)氧化表现出新的自旋态选择性,即在基态闭壳单线态上,苯乙烯被氧化成环氧化合物,而在激发态的三态和五态上,苯乙醛产物形成。
这其中,首选途径是苯乙烯被1’LBHB氧化,这是由一个速率限制的键形成耦合电子转移过程引发的,能量势垒为12.2 kcal mol-1。新生的PhIO-苯乙烯-自由基-阳离子中间体经过分子内重排生成醛。OH-/H2O配体与PhIO的碘之间的卤素键调节钴-碘基乙烯配合物1LBHB和1’LBHB的活性。这些新的机理发现丰富了人们在非血红素化学和高价碘化学方面的知识,将对新型催化剂的合理设计起到积极的作用。
据介绍,将Bronsted酸引入仿生非血红素反应可显著促进金属-氧配合物的氧化能力。然而,这种促进效应的分子机制尚不清楚。
附:英文原文
Title: Bronsted Acids Promote Olefin Oxidations by Bioinspired Nonheme CoIII(PhIO)(OH) Complexes: A Role for Low-Barrier Hydrogen Bonds
Author: Dongru Sun, Zhimin Wu, Xuan Zhang, Jindou Yang, Yufen Zhao, Wonwoo Nam, Yong Wang
Issue&Volume: March 3, 2023
Abstract: Introduction of Bronsted acids into biomimetic nonheme reactions promotes the oxidative ability of metal–oxygen complexes significantly. However, the molecular machinery of the promoted effects is missing. Herein, a comprehensive investigation of styrene oxidation by a cobalt(III)–iodosylbenzene complex, [(TQA)CoIII(OIPh)(OH)]2+ (1, TQA = tris(2-quinolylmethyl)amine), in the presence and absence of triflic acid (HOTf) was performed using density functional theory calculations. Results revealed for the first time that there is a low-barrier hydrogen bond (LBHB) between HOTf and the hydroxyl ligand of 1, which forms two valence-resonance structures [(TQA)CoIII(OIPh)(HO–--HOTf)]2+ (1LBHB) and [(TQA)CoIII(OIPh)(H2O--OTf–)]2+ (1′LBHB). Due to the oxo-wall, these complexes (1LBHB and 1′LBHB) cannot convert to high-valent cobalt–oxyl species. Instead, styrene oxidation by these oxidants (1LBHB and 1′LBHB) shows novel spin-state selectivity, i.e., on the ground closed-shell singlet state, styrene is oxidized to an epoxide, whereas on the excited triplet and quintet states, an aldehyde product, phenylacetaldehyde, is formed. The preferred pathway is styrene oxidation by 1′LBHB, which is initiated by a rate-limiting bond-formation-coupled electron transfer process with an energy barrier of 12.2 kcal mol–1. The nascent PhIO-styrene-radical-cation intermediate undergoes an intramolecular rearrangement to produce an aldehyde. The halogen bond between the OH–/H2O ligand and the iodine of PhIO modulates the activity of the cobalt–iodosylarene complexes 1LBHB and 1′LBHB. These new mechanistic findings enrich our knowledge of nonheme chemistry and hypervalent iodine chemistry and will play a positive role in the rational design of new catalysts.
DOI: 10.1021/jacs.2c12307
Source: https://pubs.acs.org/doi/10.1021/jacs.2c12307
JACS:《美国化学会志》,创刊于1879年。隶属于美国化学会,最新IF:16.383
官方网址:https://pubs.acs.org/journal/jacsat
投稿链接:https://acsparagonplus.acs.org/psweb/loginForm?code=1000
