近日,美国达特茅斯学院Robert A. Hill及其课题组发现,少突胶质细胞死亡启动同步重髓鞘化来恢复小鼠皮层髓鞘模式。这一研究成果于2023年3月16日在线发表在国际学术期刊《自然—神经科学》上。
研究人员表示,髓鞘变性发生在神经退行性疾病和衰老中。在这些条件下,常驻少突胶质细胞祖细胞(OPC)分化为少突胶质细胞,执行髓鞘修复。
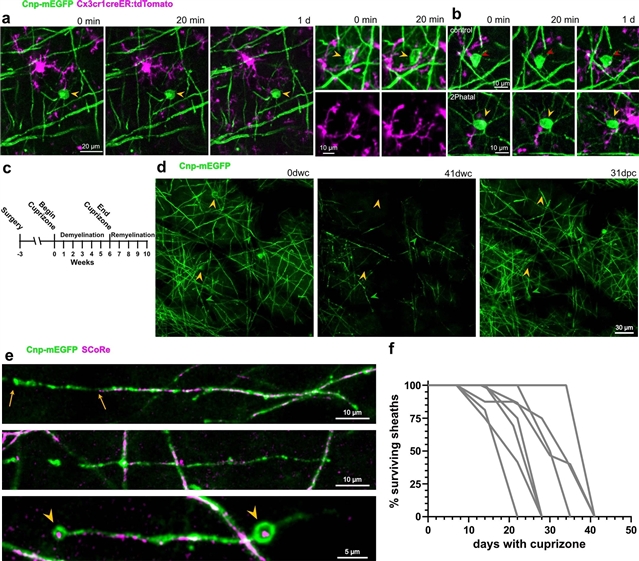
为了研究这些事件背后的细胞动力学,研究人员开发了一种非炎性脱髓鞘模型,该模型结合了活体双光子成像和单细胞消融技术,称为双光子凋亡靶向消融(2Phatal)。两种性别中少突胶质细胞的2Phatal导致髓鞘变性级联反应,并在确定的轴突上触发快速形式的同步脱髓鞘。这种脱髓鞘是由少突胶质细胞从形态上不同的、高度分支的OPC亚群分化而来。此外,再生效率取决于最初的髓鞘模式,以及生物体的年龄。
总之,通过使用2Phatal,研究人员展示了一种形式的快速同步脱髓鞘,由一个不同的OPC亚群介导,其能够在成年期恢复原始的髓鞘模式,但不会衰老。
附:英文原文
Title: Oligodendrocyte death initiates synchronous remyelination to restore cortical myelin patterns in mice
Author: Chapman, Timothy W., Olveda, Genaro E., Bame, Xhoela, Pereira, Elizabeth, Hill, Robert A.
Issue&Volume: 2023-03-16
Abstract: Myelin degeneration occurs in neurodegenerative diseases and aging. In these conditions, resident oligodendrocyte progenitor cells (OPCs) differentiate into oligodendrocytes that carry out myelin repair. To investigate the cellular dynamics underlying these events, we developed a noninflammatory demyelination model that combines intravital two-photon imaging with a single-cell ablation technique called two-photon apoptotic targeted ablation (2Phatal). Oligodendrocyte 2Phatal in both sexes results in a myelin degeneration cascade that triggers rapid forms of synchronous remyelination on defined axons. This remyelination is driven by oligodendrocytes differentiated from a subset of morphologically distinct, highly branched OPCs. Moreover, remyelination efficiency depends on the initial myelin patterns, as well as the age of the organism. In summary, using 2Phatal, we show a form of rapid synchronous remyelination, mediated by a distinct subset of OPCs, capable of restoring the original myelin patterning in adulthood but not aging.
DOI: 10.1038/s41593-023-01271-1
Source: https://www.nature.com/articles/s41593-023-01271-1
Nature Neuroscience:《自然—神经科学》,创刊于1998年。隶属于施普林格·自然出版集团,最新IF:28.771
官方网址:https://www.nature.com/neuro/
投稿链接:https://mts-nn.nature.com/cgi-bin/main.plex
