美国加州大学伯克利分校James H. Hurley等研究人员合作揭示溶酶体mTORC1–TFEB–Rag–Ragulator巨型复合物的结构。相关论文于2023年1月25日在线发表在《自然》杂志上。
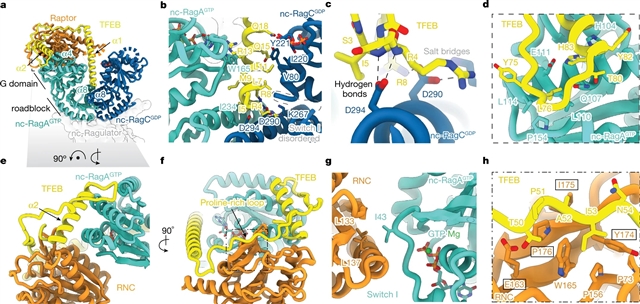
研究人员利用冷冻电子显微镜确定了雷帕霉素复合物1 的机械靶标(mTORC1)磷酸化后的TFEB结构,被称为“巨型复合物”。两个完整的Rag–Ragulator复合物将每个TFEB分子呈现到mTOR活性位点。在没有TFEB的情况下,一个Rag–Ragulator复合物以典型模式与Raptor结合。第二个Rag–Ragulator复合体(非经典)通过与第二个Ragulator复合体的依赖于RagC GDP的接触与第一个Ragulator复合体对接。非典型Rag二聚体在Rag G结构域之间的间隙中通过RagC GDP依赖的天冬氨酸钳结合TFEB的第一螺旋。在细胞中,钳突变驱动TFEB组成性地进入细胞核,而对mTORC1的定位没有影响。剩余的108个氨基酸TFEB对接域环绕Raptor,然后回到RagA。两种Rag二聚体中RagC GDP接触的双重使用解释了TFEB磷酸化对FLCN和RagC GDP状态的强烈依赖。
研究人员表示,转录因子TFEB是溶酶体生物发生和自噬的主要调节因子。mTORC1对TFEB的磷酸化是独特的mTORC1底物募集机制,该机制严格依赖于氨基酸介导的RagC GTPase激活蛋白FLCN的激活。TFEB缺乏负责招募其他mTORC1底物的TOR信号基序。
附:英文原文
Title: Structure of the lysosomal mTORC1–TFEB–Rag–Ragulator megacomplex
Author: Cui, Zhicheng, Napolitano, Gennaro, de Araujo, Mariana E. G., Esposito, Alessandra, Monfregola, Jlenia, Huber, Lukas A., Ballabio, Andrea, Hurley, James H.
Issue&Volume: 2023-01-25
Abstract: The transcription factor TFEB is a master regulator of lysosomal biogenesis and autophagy1. The phosphorylation of TFEB by the mechanistic target of rapamycin complex 1 (mTORC1)2,3,4,5 is unique in its mTORC1 substrate recruitment mechanism, which is strictly dependent on the amino acid-mediated activation of the RagC GTPase activating protein FLCN6,7. TFEB lacks the TOR signalling motif responsible for the recruitment of other mTORC1 substrates. We used cryogenic-electron microscopy to determine the structure of TFEB as presented to mTORC1 for phosphorylation, which we refer to as the ‘megacomplex’. Two full Rag–Ragulator complexes present each molecule of TFEB to the mTOR active site. One Rag–Ragulator complex is bound to Raptor in the canonical mode seen previously in the absence of TFEB. A second Rag–Ragulator complex (non-canonical) docks onto the first through a RagC GDP-dependent contact with the second Ragulator complex. The non-canonical Rag dimer binds the first helix of TFEB with a RagCGDP-dependent aspartate clamp in the cleft between the Rag G domains. In cellulo mutation of the clamp drives TFEB constitutively into the nucleus while having no effect on mTORC1 localization. The remainder of the 108-amino acid TFEB docking domain winds around Raptor and then back to RagA. The double use of RagC GDP contacts in both Rag dimers explains the strong dependence of TFEB phosphorylation on FLCN and the RagC GDP state.
DOI: 10.1038/s41586-022-05652-7
Source: https://www.nature.com/articles/s41586-022-05652-7
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
