美国莱斯大学Gao Xue团队报道了具有抗选择性的NmrA类酶催化氧化还原介导的Diels–Alder环加成。相关研究成果于2023年1月12日发表于国际顶尖学术期刊《自然—化学》。
Diels–Alder环加成是有机合成中最有力的方法之一,通常用于重要药物的合成。然而,严格控制Diels–Alder反应的立体选择性是具有挑战性的,需要通过有机催化或过渡金属策略来构建具有所需手性的复杂分子。自然界已经进化出不同类型的酶来精细地控制环化立体化学;然而,大多数报道的Diels–Alderase已被证明仅促进能量有利的非对映选择性环加成。
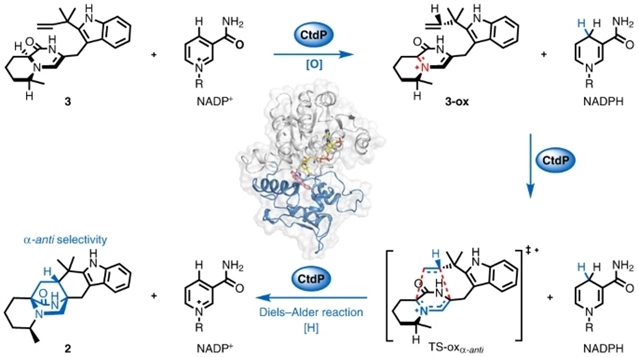
该文中,研究人员报告了CtdP的发现和表征,CtdP是一类新的双功能氧化还原酶/Diels–醛缩酶的成员,之前被注释为NmrA样转录调节器。研究证明,CtdP催化固有的不利环加成,以形成具有严格α-反选择性的双环[2.2.2]二皂辛烷支架。在计算研究的指导下,研究人员揭示了CtdP催化的逆电子需求Diels–Alder环加成的NADP+/NADPH依赖性氧化还原机制,这是利用该机制的双功能Diels–-Alder酶的第一个例子。
附:英文原文
Title: An NmrA-like enzyme-catalysed redox-mediated Diels–Alder cycloaddition with anti-selectivity
Author: Liu, Zhiwen, Rivera, Sebastian, Newmister, Sean A., Sanders, Jacob N., Nie, Qiuyue, Liu, Shuai, Zhao, Fanglong, Ferrara, Joseph D., Shih, Hao-Wei, Patil, Siddhant, Xu, Weijun, Miller, Mitchell D., Phillips, George N., Houk, K. N., Sherman, David H., Gao, Xue
Issue&Volume: 2023-01-12
Abstract: The Diels–Alder cycloaddition is one of the most powerful approaches in organic synthesis and is often used in the synthesis of important pharmaceuticals. Yet, strictly controlling the stereoselectivity of the Diels–Alder reactions is challenging, and great efforts are needed to construct complex molecules with desired chirality via organocatalysis or transition-metal strategies. Nature has evolved different types of enzymes to exquisitely control cyclization stereochemistry; however, most of the reported Diels–Alderases have been shown to only facilitate the energetically favourable diastereoselective cycloadditions. Here we report the discovery and characterization of CtdP, a member of a new class of bifunctional oxidoreductase/Diels–Alderase, which was previously annotated as an NmrA-like transcriptional regulator. We demonstrate that CtdP catalyses the inherently disfavoured cycloaddition to form the bicyclo[2.2.2]diazaoctane scaffold with a strict α-anti-selectivity. Guided by computational studies, we reveal a NADP+/NADPH-dependent redox mechanism for the CtdP-catalysed inverse electron demand Diels–Alder cycloaddition, which serves as the first example of a bifunctional Diels–Alderase that utilizes this mechanism.
DOI: 10.1038/s41557-022-01117-6
Source: https://www.nature.com/articles/s41557-022-01117-6
Nature Chemistry:《自然—化学》,创刊于2009年。隶属于施普林格·自然出版集团,最新IF:24.274
官方网址:https://www.nature.com/nchem/
投稿链接:https://mts-nchem.nature.com/cgi-bin/main.plex
